Swarts reaction
The Swarts reaction is a name reaction in organic chemistry that was first described by Frédéric Swarts in 1892 . With the help of this reaction, fluorocarbons and chlorofluorocarbons can be prepared . There are non-polar , usually aliphatic , organic polyhalides with antimony trifluoride in the presence of antimony pentachloride or chlorine partially fluorinated .
Overview reaction
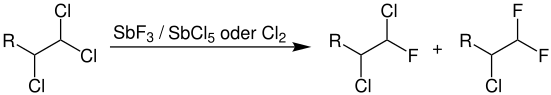
In the Swarts reaction, an organic polyhalide (here a trichloride, R = alkyl or aryl group ) is fluorinated with antimony trifluoride and antimony pentachloride or chlorine. The mixture of antimony trifluoride and chlorine is also known as Swarts reagent .
Reaction mechanism
By adding chlorine to antimony trifluoride 1 , antimony dichloride trifluoride 2 is formed . If a haloalkane (here trichloroethylsilane ) is added, the halogen atom (here a chlorine atom) is exchanged for a fluorine atom , as can be seen in FIG. 3 . With the reagents used here, the fluorinated reaction product 4 is formed .
modification
In addition to antimony trifluoride, aluminum , chromium , silver , thallium , mercury , zinc and zirconium fluorides are occasionally used. Instead of going the way over the Swarts reaction, also fluorine, and various fluorides (z. B. interhalogen compounds , xenon-fluorine compounds , hydrofluoric acid etc.) to a C = C double bond is added or C-H bonds directly fluorinated become.
application
The substitution of chlorine by fluorine using the Swarts reaction results in a lowering of the boiling point of the product, with each additional fluorine atom in the compound making an almost identical contribution to the lowering of the boiling point. This phenomenon is used, for example, in research in the field of anesthetics by fluorinating various ether compounds . The flurans obtained in this way are characterized by high vapor pressure , high stability and a low boiling point and are among the most important inhalation narcotics .
See also
Individual evidence
- ↑ a b c d e Swarts Reaction . In: Comprehensive Organic Name Reactions and Reagents . John Wiley & Sons, Inc., Hoboken, NJ, USA 2010, ISBN 978-0-470-63885-9 , pp. 2744-2747 , doi : 10.1002 / 9780470638859.conrr615 .
- ↑ Martha Windholz, Susan Budavari, Lorraine Y. Stroumtsos, Margaret Noether Done: The Merck Index . 9th edition. Merck & Co., Inc., Rahway, USA 1976, ISBN 0-911910-26-3 , pp. ONR-86-87 .
- ^ Chambers, RD (Richard D.): Fluorine in organic chemistry . [Rev. and updated ed.]. Blackwell Pub., Oxford 2004, ISBN 0-8493-1790-8 , pp. 24-26 .
- ↑ Ross C. Terrell, Louise Speers, Alex J. Szur, Thomas Ucciardi, James F. Vitcha: General anesthetics. 3. Fluorinated methyl ethyl ethers as anesthetic agents . In: Journal of Medicinal Chemistry . tape 15 , no. 6 , June 1972, p. 604-606 , doi : 10.1021 / jm00276a008 .