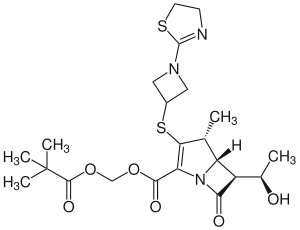
Tebipenempivoxil
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| General | |||||||||||||
| Non-proprietary name | Tebipenempivoxil | ||||||||||||
| other names |
2,2-Dimethylpropanoyloxymethyl (4 R , 5 S , 6 S ) -3- [1- (4,5-dihydro-1,3-thiazol-2-yl) azetidin-3-yl] sulfanyl-6 - [( 1 R ) -1-hydroxyethyl] -4-methyl-7-oxo-1-azabicyclo [3.2.0] hept-2-en-2-carboxylate ( IUPAC ) |
||||||||||||
| Molecular formula | C 22 H 31 N 3 O 6 S 2 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| Drug class | |||||||||||||
| properties | |||||||||||||
| Molar mass | 497.63 g · mol -1 | ||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Tebipenempivoxil is a drug that belongs to the group of carbapenems and is perorally effective. It was approved in Japan in February 2009 for the treatment of certain infectious diseases in children. Tebipenempivoxil is sold there by the Meiji Seika company under the name Orapenem . There is no approval in Europe.
pharmacology
Tebipenempivoxil is a prodrug of tebipenem, a drug from the group of β-lactam antibiotics that has a bactericidal effect by inhibiting bacterial cell wall synthesis .
Spectrum of activity
Tebipenempivoxil is approved in Japan for the treatment of children because they often tolerate oral antibiotics better than infusions. Treatment with Tebipenem is indicated for infectious diseases such as otitis media , pneumonia and sinusitis caused by pathogens such as pneumococci and Haemophilus influenza - which often develop resistance to other antibiotics .
See also
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Takao Abe, Masataka Kitamura: Process Development of Oral Carbapenem Tebipenem Pivoxil, TBPM-PI . In: Takayuki Shioiri, Kunisuke Izawa, Toshiro Konoike (eds.): Pharmaceutical Process Chemistry . Wiley-VCH, 2010, ISBN 978-3-527-63367-8 , pp. 257-272 , doi : 10.1002 / 9783527633678.ch13 .
- ↑ a b page no longer available , search in web archives: Japan Pharmaceuticals & Healthcare Report Q1 2010 . Market Report.
- ↑ M. Nakashima et al .: Effect of diet on the pharmacokinetics of tebipenem pivoxil fine granules in healthy male volunteers . In: Jpn J Antibiot. , 2009 Apr, 62 (2), pp. 136-142, PMID 19673355 .