Tellurium dichloride
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
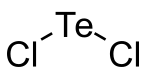
|
||||||||||
| General | ||||||||||
| Surname | Tellurium dichloride | |||||||||
| Molecular formula | TeCl 2 | |||||||||
| Brief description |
black solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 198.51 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| density |
6.9 g cm −3 |
|||||||||
| Melting point |
208 ° C |
|||||||||
| boiling point |
328 ° C |
|||||||||
| solubility |
|
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Tellurium dichloride is an inorganic chemical compound of tellurium from the group of chlorides .
Extraction and presentation
Tellurium dichloride can be obtained by reacting tellurium with dichlorodifluoromethane .
The compound can also be prepared by reacting tellurium tetrachloride with tellurium.
properties
Tellurium dichloride is an amorphous black solid that decomposes in water. It melts into a black liquid and evaporates into a purple-colored vapor.
use
Barium tellurite can be obtained by reacting barium chloride with tellurium dichloride in water .
Individual evidence
- ↑ a b c d e f g Dale L. Perry: Handbook of Inorganic Compounds . CRC Press, 2016, ISBN 978-1-4398-1462-8 , pp. 484 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ Leopold Gmelin: Tellurium: Supplement volume . Springer-Verlag, 1977, OCLC 77834357 , p. 72 (English, limited preview in Google Book search).
- ↑ EE Aynsley: 598. The preparation and properties of tellurium dichloride. In: Journal of the Chemical Society. 1953, p. 3016, doi: 10.1039 / JR9530003016 .
- ^ Arne Haaland: Molecules and Models The molecular structures of main group element compounds . OUP Oxford, 2008, ISBN 978-0-19-152860-6 , pp. 257 ( limited preview in Google Book search).
- ↑ Liv Fernholt, Arne Haaland u. a .: The molecular structure of tellurium dichloride, TeCl2, determined by gas electron diffraction. In: Journal of Molecular Structure. 128, 1985, p. 29, doi: 10.1016 / 0022-2860 (85) 85037-7 .
- ^ Richard C. Ropp: Encyclopedia of the Alkaline Earth Compounds . Newnes, 2012, ISBN 978-0-444-59553-9 , pp. 193 ( limited preview in Google Book search).
