Tetrachlorophenols
| Tetrachlorophenols | ||||||||||
| Surname | 2,3,4,5-tetrachlorophenol | 2,3,4,6-tetrachlorophenol | 2,3,5,6-tetrachlorophenol | |||||||
| other names | 2,3,4,5-TeCP |
2,3,4,6-TeCP Dowicide 6 |
2,3,5,6-TeCP |
|||||||
| Structural formula | 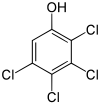 |
 |

|
|||||||
| CAS number | 4901-51-3 | 58-90-2 | 935-95-5 | |||||||
| 25167-83-3 (mixture of isomers) | ||||||||||
| PubChem | 21013 | 6028 | 13636 | |||||||
| Molecular formula | C 6 H 2 Cl 4 O | |||||||||
| Molar mass | 231.89 g mol −1 | |||||||||
| Physical state | firmly | |||||||||
| Brief description | colorless to gray crystal needles with a phenol-like odor | |||||||||
| Melting point | 69-70 ° C | 68 ° C | 113-114 ° C | |||||||
| boiling point | 164 ° C (31 mbar) | 150 ° C (21 mbar) | 288 ° C (decomposition) | |||||||
| density | 1.6 g cm −3 (80 ° C) | 1.6 g cm −3 (60 ° C) | ||||||||
| solubility | <1 g l −1 (20 ° C) | |||||||||
| poorly soluble in water | ||||||||||
|
GHS labeling |
|
|
|
|||||||
| H and P phrases | 301-315-318-335-400 | 301-319-315-410 | 301-315-318-335 | |||||||
| no EUH phrases | no EUH phrases | no EUH phrases | ||||||||
|
261-273-280-301 + 310 305 + 351 + 338 |
273-301 + 310 305 + 351 + 338-501 |
261-280-301 + 310 305 + 351 + 338 |
||||||||
The tetrachlorophenols form a group of organochlorine compounds, consisting of a benzene ring with four chlorine atoms (–Cl) and a hydroxyl group (–OH) as substituents . Their different arrangement results in three constitutional isomers with the empirical formula C 6 H 2 Cl 4 O.
Extraction and presentation
2,3,4,6-Tetrachlorophenol is considered an impurity in technical pentachlorophenol and is a by-product of its manufacture.
properties
The boiling points of the three isomers are relatively close to one another, while their melting points differ more clearly. The 2,3,5,6-tetrachlorophenol, which has the highest symmetry, has the highest melting point. The tetrachlorophenols are sparingly soluble in water and react slightly acidic in solution.
use
2,3,4,6-Tetrachlorophenol is used in the manufacture of pharmaceuticals, fertilizers and pesticides and is used as a wood preservative. Sodium 2,3,5,6-tetrachlorophenolate (the sodium salt of 2,3,5,6-tetrachlorophenol, CAS number: 85712-07-8) is used as a fungicide .
safety instructions
The decomposition of tetrachlorophenols produces hydrogen chloride and other highly toxic chlorine products such as PCDD / PCDF. The compounds are very toxic to aquatic organisms.
literature
- Jennifer M. Gee, JL Peel: Metabolism of 2,3,4,6-Tetrachlorophenol by Micro-organisms from Broiler House Litter . In: Microbiology . tape 85 , no. 2 , 1974, p. 237–243 , doi : 10.1099 / 00221287-85-2-237 (free full text).
Individual evidence
- ↑ a b c d e f Entry on 2,3,4,5-tetrachlorophenol in the GESTIS substance database of the IFA , accessed on October 14, 2012(JavaScript required) .
- ↑ a b c d e Entry on 2,3,4,6-tetrachlorophenol in the GESTIS substance database of the IFA , accessed on October 14, 2012(JavaScript required) .
- ↑ a b c d Entry on 2,3,5,6-tetrachlorophenol in the GESTIS substance database of the IFA , accessed on October 14, 2012(JavaScript required) .
- ↑ Data sheet 2,3,4,6-tetrachlorophenol from Sigma-Aldrich , accessed on July 1, 2011 ( PDF ).
- ↑ 2,3,4,6-tetrachlorophenol (Enius)
- ↑ 2,3,4,6-tetrachlorophenol (SpecLab)


