Tinzaparin
| Structural formula | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|||||||||
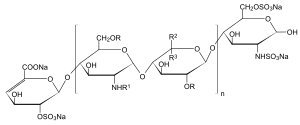
| n = 1 to 25, R = H or SO 3 Na, R 1 = H, SO 3 Na or COCH 3 , R 2 = H and R 3 = COONa or R 2 = COONa and R 3 = H | |||||||||
| General | |||||||||
| Surname | Tinzaparin | ||||||||
| CAS number |
|
||||||||
| Monomers / partial structures | substituted disaccharide | ||||||||
| Qualitative molecular formula |
unspecific |
||||||||
| Molar mass estimation |
6500 daltons (tinzaparin sodium, average) |
||||||||
| ATC code | |||||||||
| properties | |||||||||
| safety instructions | |||||||||
|
|||||||||
| Toxicological data |
550,000 IU / 24h (= 7,930 IU / kg / 24h) ( LD Lo , human , iv ) |
||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||
Tinzaparin (generic name for the medicinally used sodium salt: tinzaparin sodium ) is a blood coagulation inhibitor and is one of the group of low molecular weight heparins . It is used to prevent and treat deep vein thrombosis and pulmonary embolism and is given by subcutaneous injection. Tinzaparin is also used for hemodialysis in the extracorporeal circuit.
Tinzaparin sodium is the sodium salt of the active substance, which is obtained by enzymatic depolymerization (= splitting) of unfractionated heparin chains using heparinase . Heparinase is obtained from Flavobacterium heparinum . Tinzaparin sodium is manufactured by the Danish company LEO Pharma and marketed worldwide as an injection solution under the trade name innohep . The drug requires a prescription.
pharmacology
Tinzaparin has an inhibitory effect on various coagulation factors in the coagulation cascade . The coagulation factor X and the coagulation factor II are inhibited via antithrombin . In addition, the tissue factor is inhibited by the release of Tissue Factor Pathway Inhibitor (TFPI) . As a result, less thrombin is produced and this inhibits the production of a fibrin network. The result is decreased blood clotting.
The potency of Tinzaparin is determined using the anti- factor Xa determination and expressed in anti-Xa international units (IU).
The overall effect of a low molecular weight heparin cannot be measured directly. Anti-Xa and anti-IIa measurements are used as a temporary surrogate biomarker for low molecular weight heparins. A measurement of the anti-Xa level is usually not carried out in everyday clinical practice, as this measurement does not correlate directly with the effect in the body.
The average molar mass is between 5,500 and 7,500 Daltons (median value = 6,500 Daltons).
The table summarizes the pharmacokinetic properties of tinzaparin determined on the basis of anti-Xa measurements (mean value and standard deviation are given in each case):
| Parameter (unit) | Multiple dosing (n = 21) | Single dosage (n = 14) | |
|---|---|---|---|
| Day 1 175 IU / kg |
Day 5 175 IU / kg |
4,500 IU * | |
| C max (IU / ml) | 0.87 (0.15) | 0.93 (0.15) | 0.25 (0.05) |
| T max (h) | 4.4 (0.7) | 4.6 (1.0) | 3.7 (0.9) |
| AUC 0-∞ (IE h / ml) | 9.0 (1.1) | 9.7 (1.4) | 2.0 (0.5) |
| T 1/2 (h) | 3.3 (0.8) | 3.5 (0.6) | 3.4 (1.7) |
- * corresponding to an average of 64.3 IU / kg
Indications
In Germany, Tinzaparin is approved for the following areas of application:
- Postoperative thrombosis prophylaxis for surgical interventions in the medium and low risk range, including accompanying dispositional risk factors
- Therapy of thromboembolic events including deep vein thrombosis and pulmonary embolism
- Use in the extracorporeal circuit during hemodialysis
In other EU countries, there are additional approvals for the following areas of application:
- Postoperative thrombosis prophylaxis at high risk (e.g. hip and knee joint operations)
- Immobilization due to severe internal diseases
development
The clinical development of tinzaparin led to approval in the prophylactic field after surgical interventions in 1994. In 1997, Tinzaparin was approved in Germany as the first representative of low molecular weight heparins for the initial therapy of deep vein thrombosis, and in 1999 for the acute treatment of pulmonary embolism in hemodynamically stable patients (up to the beginning of stage III).
The current clinical development of the substance focuses on the one hand on the long-term (6-month) use of tinzaparin after acute leg vein thrombosis or pulmonary embolism (secondary prophylaxis) and on the other hand on its use in patients with age-related impaired renal dysfunction.
Practical features
In all areas of application, Tinzaparin is injected subcutaneously only once a day. This also applies in particular to use in the therapeutic area.
Tinzaparin does not accumulate in elderly patients with impaired renal function and does not lead to an increased bleeding tendency in patients 70 years of age or older compared to the aPTT-adjusted unfractionated heparin administration. No dose adjustment is necessary in these patients. This prevents a possible loss of effectiveness of Tinzaparin.
Contraindications and restrictions on use
Like other low molecular weight heparins, Tinzaparin must not be used in the event of hypersensitivity to the active ingredient, in coagulation disorders such as the presence of a reduced number of blood platelets (thrombocytopenia) or a lack of coagulation factors, in the event of a tendency to bleed due to ulcers or other organ changes and injuries, as well as septic endocarditis .
Therapeutic doses (175 IU / kg) are contraindicated in patients receiving neuraxial anesthesia . The drug should be discontinued at least 24 hours before such a planned procedure and administration should be continued no earlier than 4–6 hours after anesthesia or removal of the catheter.
The use of tinzaparin in patients with creatinine clearance below 30 ml / min is not recommended as no dose has been established for this patient population. The available data shows that with a creatinine clearance of 20 ml / min or higher there is no accumulation of the active substance. If necessary, tinzaparin treatment in these patients can be administered with caution under anti-Xa surveillance. Even if anti-Xa monitoring, which is carried out by means of the chromogen assay, is only of limited suitability for predicting a risk of bleeding, it is nevertheless best suited for measuring the pharmacodynamic effect. If necessary, the dose should be adjusted based on anti-factor Xa activity.
Web links
- Data sheet TINZAPARIN SODIUM CRS (PDF) at EDQM , accessed on July 30, 2017.
Individual evidence
- ^ A b Tinzaparin Full Prescribing Information USA .
- ^ European Pharmacopoeia, 6th Edition, 2008 .
- ↑ Data sheet Heparin sodium salt from porcine intestinal mucosa from Sigma-Aldrich , accessed on July 30, 2017 ( PDF ).
- ↑ HA Spiller: Heparin and Low-Melecular-Weight Heparins. In RC Dart: Medical toxicology. 3. Edition. Lippincott Williams & Wilkins, 2004, ISBN 0-7817-2845-2 , pp. 618ff.
- ↑ a b Technical information for innohep 20,000 Anti-Xa IU / ml injection solution , as of September 2016.