Tiotropium bromide
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
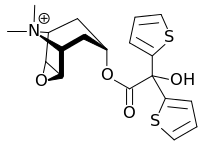
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Tiotropium bromide | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 19 H 22 BrNO 4 S 2 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 472.42 g mol −1 | |||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Tiotropium bromide , shortly tiotropium , is a polycyclic quaternary ammonium compound , as anticholinergic ( antimuscarinic ) and bronchodilator for patients with chronic obstructive pulmonary disease ( COPD is used). Tiotropium is made available for inhalation in two different inhalers (HandiHaler, Respimat). In the Respimat, tiotropium can be used not only for COPD but also for the treatment of bronchial asthma in adult patients who receive a combination of inhaled corticosteroids (≥ 800 μg budesonide / day or equivalent) and long-acting beta2 agonists as long-term therapy, and who received the previous year have experienced at least one severe exacerbation. The short name is tiotropium.
Clinical information
Tiotropium bromide is structurally derived from scopolamine and, like ipratropium bromide, is a quaternary ammonium salt. This results in a reduced blood-brain barrier penetration, whereby the typical central side effects of anticholinergic active ingredients are reduced. Compared to ipratropium bromide, tiotropium bromide has a longer half-life, which means that it can be administered once a day. It takes effect about 30 minutes after inhalation. Tiotropium bromide is 20 to 25% metabolized; 75 to 80% are excreted renally unchanged .
development
Tiotropium bromide was developed by Boehringer Ingelheim in 1991 . In 2005 it received the Robert Koch Award, presented by the journal MMW-progress in medicine .
Side effects
Typical side effects derive from the anticholinergic effect. Dry mouth is common; glaucoma , indigestion and urinary retention are much less common .
In 2008 , a meta-analysis was carried out on the basis of observations which suggest that the use of drugs containing tiotropium bromide could promote the development of heart attacks and strokes . The authors of this study concluded that the relative risk of such side effects would increase by 50%. The results of the meta-analysis were in some cases questioned by experts with reference to alleged methodological deficiencies. One week after the publication of this retrospective observation, the results were put into perspective by the largest prospective study on tiotropium to date. In the 4-year UPLIFT study (Understanding Potential Long-term Impacts on Function with Tiotropium), the primary endpoint of demonstrating a slowdown in disease progression with tiotropium was not achieved. However, with regular use of the drug there was no increased cardiovascular event. However, this study was carried out exclusively by employees of the manufacturing company; the statistical analysis was also carried out by the manufacturing company alone. A conflict of interest is therefore likely. In 2011, another meta-analysis was published that showed an increased cardiovascular mortality after inhalation via Respimat. Overdosing with this inhalation technique was suspected to be the cause of the increased mortality, as a higher concentration of active substance was achieved both in the airways and in the blood. The tolerability of the substance when inhaled using Respimat was investigated in a study published in 2013 on 17,135 patients with COPD. At the approved dosage of 5 µg daily, the Respimat showed a safety profile comparable to the HandyHaler with the same effectiveness.
Benefit assessment
The German Institute for Quality and Efficiency in Health Care (IQWiG) regards it as proven that tiotropium has an additional benefit compared to the group of long-acting β-adrenoceptor agonists , both in terms of the frequency of exacerbations and the need for hospital stays caused by exacerbations.
Trade names
Srivasso (D), Braltus (D), Spiriva (D).
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ JC Frölich: Practical medicinal therapy. Springer, 2003, ISBN 978-3-540-01025-8 .
- ↑ Sonal Singh, Yoon K. Loke, Curt D. Furberg: Inhaled Anticholinergics and Risk of Major Adverse Cardiovascular Events in Patients With Chronic Obstructive Pulmonary Disease , JAMA , 2008; 300 (12): 1439-1450. doi : 10.1001 / jama.300.12.1439 .
- ↑ Stephen J. Senn: Overstating the evidence - double counting in meta-analysis and related problems , BMC Med Res Methodol , 2009; 9:10 , doi : 10.1186 / 1471-2288-9-10 ; PMC 2653069 (free full text).
- ↑ DP Tashkin, B. Celli, S. Senn, D. Burkhart, S. Kesten, S. Menjoge, M. Decramer: A 4-Year Trial of Tiotropium in Chronic Obstructive Pulmonary Disease , New Engl. J. Med. , 2008 , 359, 1543–1554, doi : 10.1056 / NEJMoa0805800 (free full text).
- ↑ S. Singh, YK Loke et al. a .: Mortality associated with tiotropium mist inhaler in patients with chronic obstructive pulmonary disease: systematic review and meta-analysis of randomized controlled trials , BMJ (Clinical research ed.) , Volume 342, 2011, p. d3215, PMID 21672999 . PMC 3114950 (free full text).
- ↑ Cardiac risks of COPD therapy with the Respimat inhaler. Archived from the original on January 18, 2013. In: aerzteblatt.de . June 15, 2011. Retrieved August 23, 2012.
- ^ Robert A. Wise, Antonio Anzueto, Daniel Cotton, Ronald Dahl, Theresa Devins, Bernd Disse, Daniel Dusser, Elizabeth Joseph, Sabine Kattenbeck, Michael Koenen-Bergmann, Gordon Pledger, Peter Calverley: Tiospir: Tiotropium Respimat Inhaler and the Risk of Death in COPD , New Engl. J. Med. , 2013, 369, 1491–1501, doi : 10.1056 / NEJMoa1303342 (free full text).
- ↑ Tiotropium has benefits for patients with COPD , August 22, 2012.
- ↑ apotheke-adhoc.de: Srivasso = Spiriva ≠ Braltus , accessed on November 13, 2017.