Trimethylaniline
The trimethylanilines form a group of substances in chemistry and are aromatic compounds with three methyl groups (-CH 3 ) and one amino group (-NH 2 ) as substituents on the benzene ring (" aromatic amines "). The different arrangement of the substituents results in six constitutional isomers with the empirical formula C 9 H 13 N. If the substance group is expanded so that methyl groups are also located on the nitrogen atom, further isomers result, among others. a. the N , N -dimethyltoluidines .
| Trimethylaniline | ||||||||||
| Surname | 2,3,4-trimethylaniline | 2,3,5-trimethylaniline | 2,3,6-trimethylaniline | 2,4,5-trimethylaniline | 2,4,6-trimethylaniline | 3,4,5-trimethylaniline | ||||
| other names | 1-amino-2,4,5-trimethylbenzene (2,4,5-trimethylbenzene) amine psi-cumidine pseudocumidine |
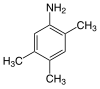
Mesidine 2-aminomesitylene 2-amino-1,3,5-trimethylbenzene |
||||||||
| Structural formula |

|

|

|
|

|

|
||||
| CAS number | 1467-35-2 | 137-17-7 21436-97-5 ( hydrochloride ) |
88-05-1 6334-11-8 (hydrochloride) |
1639-31-2 | ||||||
| Trimethylaniline-cas (mixture of isomers) | ||||||||||
| PubChem | 73844 | 12782408 | 11958947 | 8717 | 6913 | 74227 | ||||
| Molecular formula | C 9 H 13 N | |||||||||
| Molar mass | 135.21 g mol −1 | |||||||||
| Physical state | firmly | liquid | ||||||||
| Brief description | light yellow to red-brown solid |
yellow-brown liquid with a characteristic odor |
||||||||
| Melting point | 68 ° C | −5 ° C | ||||||||
| boiling point | 234 ° C | 232-234 ° C | ||||||||
| density | 0.957 g cm −3 | 0.96 g cm −3 | ||||||||
| solubility | practically insoluble in water | |||||||||
|
GHS labeling |
|
|
||||||||
| H and P phrases | 350-331-311-301-411 | 330-302-312-315-319-335 | ||||||||
| no EUH phrases | no EUH phrases | |||||||||
| ? |
260-284-305 + 351 + 338 320-405-501 |
|||||||||
use
2,4,6-Trimethylaniline is used in the manufacture of azo polymerization initiators and is an intermediate in the manufacture of dyes and pharmaceuticals. 2,4,5-trimethylaniline is used in the production of the red dye Ponceau 3R .
Individual evidence
- ↑ a b c d e Entry on 2,4,5-trimethylaniline in the GESTIS substance database of the IFA , accessed on January 31, 2012(JavaScript required) .
- ↑ a b c d e f Entry on 2,4,6-trimethylaniline in the GESTIS substance database of the IFA , accessed on January 31, 2012(JavaScript required) .
- ↑ National Institute of Environmental Health Sciences: Abstract for TR-160 - 2,4,5-Trimethylaniline (CASRN 137-17-7) , accessed November 18, 2014.


