Tryparsamide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
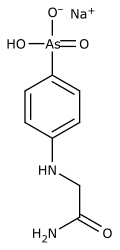
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Tryparsamide | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula |
|
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| Drug class |
Antiprotozoic |
|||||||||||||||
| properties | ||||||||||||||||
| Molar mass | ||||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Tryparsamide is a chemotherapeutically effective arsenic compound that was developed in the United States in 1915 by Walter Abraham Jacobs and Michael Heidelberger at the New York Rockefeller Institute for Medical Research .
From 1922 the substance was used in drugs in Africa for the treatment of sleeping sickness caused by Trypanosoma brucei , especially in advanced and chronic cases, but from 1934 only in combination with other active ingredients such as suramin . Because of its good solubility in water , it could be administered intramuscularly as well as intravenously . After the Second World War , it was replaced by melarsoprol in most countries with the exception of Nigeria , where it was in use until the early 1970s, because of the increasing development of resistance and sometimes serious side effects such as blindness and damage to the optic nerve .
In the United States and some other countries, tryparsamide was also used temporarily as a remedy for the neurosyphilis caused by Treponema pallidum .
literature
- Stéphane Gibaud, Gérard Jaouen: Tryparsamide. In: Gérard Jaouen: Medicinal Organometallic Chemistry. Series: Topics in Organometallic Chemistry . Volume 32.Springer , Berlin and Heidelberg 2010, ISBN 3-64-213184-0 , p. 5/6
- Arsphenamines. In: Walter Sneader: Drug Discovery: A History. John Wiley and Sons, 2005, ISBN 0-47-189979-8 , pp. 49–56 (on tryparsamide in particular pp. 54/55)
- KDB Thomson: Tryparsamide. In: The Lancet . Volume 334, Edition 8662 of September 2, 1989, p. 573 (on experiences from practical use in Africa)
- Margitta Albinus: Hager's handbook of pharmaceutical practice. 9: substance P - Z . Springer-Verlag, Berlin and others 1993, ISBN 3-540-52688-9 , pp. 1108 f . ( limited preview in Google Book search).
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.