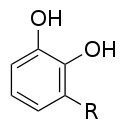
Urushiole
Urushiole are a group of solid or oil-like chemical compounds that are found in sumac plants such as poison sumac Toxicodendron quercifolium , especially in various Rhus species such as poison ivy ( Toxicodendron radicans , English poison ivy ), poison oak ( Toxicodendron diversilobum , English poison oak , also Toxicodendron oak in the American-speaking area quercifolium ) and lacquer tree ( Rhus verniciflua ) occur. The group of fabrics takes its name from its occurrence in the resin of the lacquer tree, which is also known as urushi , a Japanese handicraft .

In 1906, Kisaburo Miyama (* 1873) isolated a compound from the lacquer tree Rhus vernicifera in Japan , which he called urushiole . At the beginning of the 20th century, Majima Rikō examined the compounds more closely and realized that they are catechol derivatives.
Structure, representatives and properties

Chemically, urushiols are derivatives of 1,2-dihydroxybenzene ( pyrocatechol ). A saturated respectively to the central catechol alkyl ( 3-Alkylbrenzcatechine ) or unsaturated alkenyl - substituent ( 3-Alkenylbrenzcatechine bound). In the poisonous plants there is always a mixture of different urushiols, whereby - depending on the plant species, urushiol I and III usually make up the main component. About 15 substances were identified, the saturated urushiol I is the only solid substance. The mixture used as lacquer and extracted from the lacquer tree Toxicodendron vernicifluum is a yellow, oily liquid with a boiling point of 200-210 ° C.
All urushiols are only minimally soluble in water, on the other hand well in alcohols and diethyl ether . The unsaturated representatives II to V tend to polymerize . Solutions in methanol or ethanol are oxidized by atmospheric oxygen to the corresponding ortho- quinones even at room temperature .
Biological properties
Urushiols are extremely potent allergens ; by urushiols from poison ivy ( poison ivy ) and -oak ( Poison oak ) induced contact allergies are the most common form of allergy in the United States . The toxic effect of the substances is based on the inhibition of prostaglandin biosynthesis. In vitro studies with preparations from sheep - seminal vesicles exhibited even at low concentrations (37 mol / l of an extract of Rhus radicans ) a complete inhibition of prostaglandins.
The allergy and the triggered contact dermatitis depend on the chemical structure of the urushiole. In North America , where most of the cases are described, it has been observed that these skin reactions depend on the degree of saturation of the alkyl groups . In most individuals, urushiols with at least two double bonds are allergenic , and less than half react to urushiols with only saturated alkyl groups. This is a real allergy that is mediated via antigen-presenting T lymphocytes . The reaction only occurs with prior sensitization) . This allergy can be triggered in 50 to 70% of the North American population.
Web links
- Giftpflanze.com: Urushiol
- Entry for Urushiol V in the ChemSpider database of the Royal Society of Chemistry
Individual evidence
- ↑ a b Entry on Urushiole. In: Römpp Online . Georg Thieme Verlag, accessed on June 8, 2014.
- ^ A b c Donald G. Crosby: The poisoned weed: plants toxic to skin. Oxford University Press, 2004, ISBN 978-0-1951-5548-8 , pp. 97-98.
- ↑ Peter Altmeyer, Martina Bacharach-Buhles: Springer Encyclopedia Dermatology, Allergology, Environmental Medicine. Springer, 2002, ISBN 978-3-540-41361-5 , p. 585.
- ^ R. Hänsel, K. Keller, H. Rimpler, G. Schneider: Hagers Handbook of Pharmaceutical Practice. Volume 6: Drugs P-Z , 5th edition, Springer, 1994, ISBN 978-3-540-52639-1 , pp. 456-463.
- ↑ Phillip M. Williford, Elizabeth F. Sherertz: poison ivy dermatitis, Nuances in Treatment. In: Arch. Fam. Med. 1994; 3: 184-188; PMID 7994440 .
- ↑ RS Kalish, JA Wood, A. LaPorte: Processing of urushiol (poison ivy) hapten by both endogenous and exogenous pathways for presentation to T cells in vitro. In: J. Clin. Invest. 1994; 93 (5): 2039-2047; doi : 10.1172 / JCI117198 .