Vertico-SMI
The Vertico-SMI is a fluorescence microscope for the three-dimensional recording of cells in the nanometer range (super resolution microscopy). In contrast to comparable approaches, the marking is carried out with normal fluorescent dyes such as GFP, Cy2 / 3, fluorescein, alexa and atto dyes, based on the so-called blinking phenomenon. It is based on two microscope technologies, which were developed in 1996, the localization microscopy SPDM and the structured illumination SMI. The effective optical resolution of this optical nanoscope reaches 5 nm in 2D and 40 nm in 3D and is therefore significantly better than the physical resolution limit of 200 nm, postulated by Abbe's law in 1873.
configuration
"SMI" stands for a special type of laser-optical illumination ( Spatially Modulated Illumination , Spatially Structured Illumination) and "Vertico" for the vertical arrangement of the microscope axis, which enables fixed cells as well as living cells with a three-dimensional effective optical resolution of 40 Nanometer (1 nanometer = 1 nm = 10 −9 m) to be analyzed.
Basics
The microscope was developed by Christoph Cremer , Professor for Applied Optics and Information Processing at Heidelberg University / Institute for Molecular Biology . It is based on a combination of light-optical techniques of localization microscopy (SPDM, Spectral Precision Distance Microscopy ) and structured illumination (SMI, Spatially Modulated Illumination ). A special feature in contrast to focusing techniques such as 4Pi microscopy are the wide-field images in which the entire sample is illuminated and detected at the same time. This enables whole cells to be quickly recorded using the nanoscopic technique. Only 2 minutes are required for 3D recordings of such whole cells with typical image fields of size 20 µm × 20 µm.
The effective optical resolution of this optical nanoscope has reached a range of 5 nm in 2D and 40 nm in 3D and is therefore significantly below the physical limit of 200 nm, which, according to Abbe's law in 1873, is the physical limit below which a light microscopic resolution theoretically not possible has been postulated.
Spatially Modulated Illumination (SMI)
Spatially Modulated Illumination (SMI) stands for spatially structured lighting. SMI microscopy is a light-optical process known as point spread function engineering. This is understood to mean methods that modify the point spread function (PSF) of a microscope in a suitable manner in order to either increase the optical resolution, the precision of distance measurements on point-shaped, i.e. i.e., to maximize fluorescent objects that are small compared to the wavelength or to extract other structural parameters in the nanometer range.
With the SMI microscope currently being developed at the Kirchhoff Institute for Physics at the University of Heidelberg, this is achieved by the fact that the excitation intensity in the object space is not homogeneous, in contrast to conventional wide-field fluorescence microscopes, but is spatially precisely modulated in the axial direction by using two opposing, interfering laser beams becomes. The principle of the spatially modulated wave field was developed in 1993 by Bailey et al. developed. With the Heidelberg SMI microscopy approach, the object is moved in high-precision steps through the wave field or the wave field itself is shifted (phase). This results in an increase in the axial size and distance resolution.
SMI can resolution microscopy technologies, such as 3D LIMON or LSI with other Super TIRF as a total reflection are (TIRF) interferometer with spatially structured illumination, combined. In combination with TIRF, light-optical images of autofluorescent (self-luminous) structures in human eye tissue can be recorded with unprecedented optical resolution using three different excitation wavelengths (488, 568 and 647 nm). The optical resolution is 100 nm when examining the macular degeneration of diseased human eye tissue.
Spectral Precision Distance Microscopy (SPDM)
SPDM (Spectral Precision Distance Microscopy) is a light-optical method of fluorescence microscopy with which position, distance and angle measurements are possible on “optically isolated” particles (e.g. individual molecules) far below the optical diffraction limit . “Optically isolated” means that at a certain point in time only a single particle / molecule is registered in an area determined by conventional optical resolution (typically approx. 200–250 nm in diameter ). This is possible if the particles / molecules located in such an area bear different spectral markings (e.g. have different colors or show other useful differences in the light emission).
The structure resolution possible with SPDM can be specified by the smallest measurable distance between two “punctiform” particles with different spectral markings determined in their spatial position (“topological resolution”). Simulation calculations have shown that under suitable assumptions about localization accuracy, particle density, etc., the "topological resolution" corresponds to a spatial frequency that is equivalent to a greatly increased optical resolution in the sense of the classic definition.
It is a localization microscopy, which makes an effective optical resolution possible, which is many times better than the conventional optical resolution (approx. 200-250 nm), given by the half- width of the main maximum of the effective point spread function. Using suitable laser-optical precision methods, positions and distances are measured with nanometer accuracy between target objects with different spectral signatures that are considerably smaller than the half-width of the point spread function. An important area of application is genome research (studying the functional organization of the genome). Another important area of application is membrane structure research.
SPDMphymod “Flashing dyes” instead of photoswitchable molecules
Cremer's working group found out in 2008 that under certain photophysical conditions, super resolution microscopy can also be carried out for many “very common” dye molecules such as GFP or Alexa dyes. This means that luminous molecules can be used in the same spectral color (but with different spectral signatures due to “blinking” properties). By combining many thousands of individual images of the same cell, “localization images” with significantly improved optical resolution are obtained with the help of laser-optical precision measurements.
This extends the applicability of the SPDM method to numerous areas of biophysical, cell biological and medical research.
- Super resolution microscopy - localization microscopy
LIMON: 3D Super Resolution Microscopy
LIMON (Light MicrOscopical nanosizing microscopy) was developed in 2001 at the University of Heidelberg and combines the two methods localization microscopy and structured lighting to create 3D super resolution microscopy.
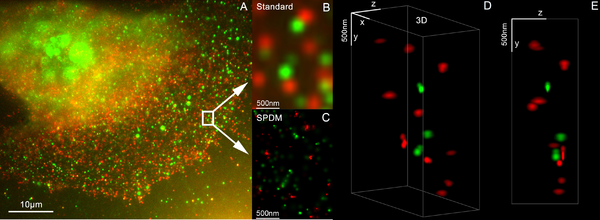
The procedure is as follows: first SMI recordings are made and then the SPDM process is carried out. The SMI process determines the center of the particles and their propagation in the direction of the microscopic axis. While the center of the particles / molecules can be determined with an accuracy of 1–2 nm, the propagation at this point can be determined up to an axial diameter of approx. 30–40 nm. The lateral position of the individual particles / molecules is then determined with SPDM with an accuracy of a few nanometers. SPDM currently achieves 16 images per second (depending on the camera) with an effective resolution of 5 nm in 2D (object plane); around 2000 such images are combined with SMI data in order to achieve a three-dimensional image of the highest possible resolution (effective optical 3D resolution approx. 40–50 nm).
With this two-color colocalization 3D super resolution microscopy, the spatial arrangement of the two genes Her2 / neu and HER3 active in breast cancer was determined with an accuracy of about 25 nm, and their cluster formation, which is probably relevant for cancer development, was analyzed at the single-molecule level.
Biomolecular machines, highly complex nanostructures that fulfill fundamental functions in the body's cells, can also be examined in their biologically relevant composition with the 3D Super Resolution Microscopy LIMON. Individual proteins or nucleic acids that are hidden in the 3-D molecular complex of so-called biomolecular machines can be made visible by variable marking without destroying the complex.
Individual evidence
- ↑ a b Jürgen Reymann, David Baddeley, Manuel Gunkel, Paul Lemmer, Werner Stadter, Thibaud Jegou, Karsten Rippe, Christoph Cremer, Udo Birk: High-precision structural analysis of subnuclear complexes in fixed and live cells via spatially modulated illumination (SMI) microscopy . In: Chromosome Research . tape 16 , no. 3 , May 2008, p. 367-382 , doi : 10.1007 / s10577-008-1238-2 , PMID 18461478 .
- ↑ G Best, R Amberger, D Baddeley, T Ach, S Dithmar, R Heintzmann, C Cremer: Structured illumination microscopy of autofluorescent aggregations in human tissue . In: Micron , 42, 2011, pp. 330-335
- ↑ Manuel Gunkel, Fabian Erdel, Karsten Rippe, Paul Lemmer, Rainer Kaufmann, Christoph Hörmann, Roman Amberger, Christoph Cremer: Dual color localization microscopy of cellular nanostructures . In: Biotechnology Journal . tape 4 , no. 6 , 2009, ISSN 1860-6768 , p. 927-938 , doi : 10.1002 / biot.200900005 .
- ^ D Baddeley, C Batram, Y Weiland, C Cremer, UJ. Birk: Nanostructure analysis using Spatially Modulated Illumination microscopy . In: Nature Protocols , 2007, 2 :, pp. 2640-2646
- ^ Rainer Kaufmann, Patrick Müller, Georg Hildenbrand, Michael Hausmann, Christoph Cremer: Analysis of Her2 / neu membrane protein clusters in different types of breast cancer cells using localization microscopy . In: Journal of Microscopy , 2010, doi: 10.1111 / j.1365-2818.2010.03436.x .
Web links
- Heidelberg Innovation Forum 2009: The world's fastest light microscope named best business idea
- GFP Superresolution (PDF; 330 kB)
- List of publications on light-optical nanoscopy
- Press release from Heidelberg University
- "Flashing dyes". Ruperto Carola , research journal from the University of Heidelberg