Von Braun Rudolph synthesis
The Von Braun Rudolph synthesis is a name reaction in organic chemistry that was discovered by the two German chemists Julius von Braun ( Heidelberg ) and Walter Rudolph ( Frankfurt am Main ) at the end of the 1930s. This was based on 30-year-old studies. After von Braun's death, her work was published in the reports of the German Chemical Society in 1941 . It is used for the synthesis of tetrazole derivatives and is therefore also known as the Von-Braun-Rudolph tetrazole synthesis.
description
A are in this case reacted imide chloride and hydrazoic acid which form the tetrazole derivative at room temperature.
In order to avoid side reactions - such as the formation of urea or aniline tetrazole - sodium azide is often added in an aqueous phosphate buffer together with a water-miscible organic solvent (for example acetone ).
Reaction mechanism
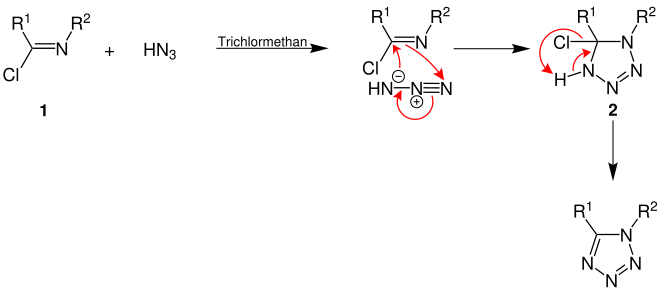
The mechanistic details of the Von Braun-Rudolph synthesis are not yet known. The following graphic shows an assumed reaction mechanism for the synthesis of tetrazole derivatives:
First, a 1,3-dipolar cycloaddition takes place between the imide chloride ( 1 ) disubstituted with two organic radicals (R 1 and R 2 ) and the hydrazoic acid with formation of 2 . Then hydrogen chloride is eliminated so that the aromatic tetrazole ring is formed and the tetrazole derivative is formed.
Individual evidence
- ↑ Julius von Braun, Walter Rudolph: Syntheses in the Tetrazol series (II). Message. In: Reports of the German Chemical Society. 74, 1941, pp. 264-272, doi: 10.1002 / cber.19410740217 .
- ↑ a b Zerong Wang (Ed.): Comprehensive Organic Name Reactions and Reagents . 3 volume set. John Wiley & Sons, Hoboken, NJ 2009, ISBN 978-0-471-70450-8 , pp. 2904-2907 .