Zibotentan
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
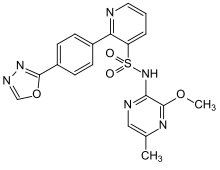
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Zibotentan | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 19 H 16 N 6 O 4 S | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 424.433 g · mol -1 | |||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Zibotentan is a potential orally administered cytostatic agent for the treatment of hormone-resistant prostate cancer . It is currently in phase 3 clinical trials .
Zibotentan is being developed by the British pharmaceutical company AstraZeneca . The ethanolamine salt is used medicinally .
Mechanism of action
Zibotentan is a selective endothelin A receptor antagonist . Its plasma half-life is 8 hours and the plasma protein binding is 73.8%.
literature
- AstraZeneca, ZD4054 information to support Pre-clinical Studies: AstraZeneca, 7pp., 2005
- CD.Morris, A.Rose, J.Curwen, AM.Hughes, DJ.Wilson, DJ.Webb. Specific inhibition of the endothelin A receptor with ZD4054: clinical and pre-clinical evidence. Br J Cancer .92,2005
- WR.Schelman, G.Liu, G.Wilding, T.Morris, D.Phung, R. Dreicer. Investigational New Drugs , A phase I study of zibotentan (ZD4054) in patients with metastatic, castrate-resistant prostate cancer. DOI , 10.1007 / s10637-009-9318-5.2009
Individual evidence
- ↑ There is not yet a harmonized classification for this substance . A labeling of N- (3-methoxy-5-methyl-2-pyrazinyl) -2- [4- (1,3,4-oxadiazol-2-yl) phenyl] -3- is shown, which is derived from a self-classification by the distributor pyridinesulfonamide in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 10, 2020.
- ↑ Zibotentan data sheet from Sigma-Aldrich , accessed on February 10, 2020 ( PDF ).