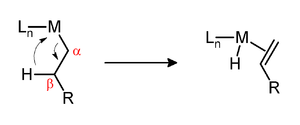
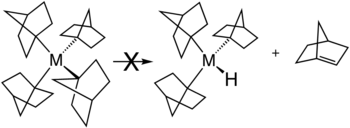β-hydride elimination
A β-hydride elimination is a reaction in which an alkyl group bound to a metal center reacts to form the alkene with the elimination of a metal hydride . A prerequisite for β-hydride elimination is the existence of a hydrogen atom in the β position . For this reason, for example, complexes with n- butyl and tert-butyl ligands can react in this form, but not those with methyl and neo- pentyl ligands . Furthermore, the metal center must have a free binding site in the cis position to the alkyl group.
On the one hand, β-hydride elimination can be an important reaction step in a synthesis, but on the other hand it is often an undesirable side reaction. For example, the SHOP process relies on β-hydride elimination to produce α-alkenes, which are then used to make detergents . The preparation of branched polymers from ethene is based on the chain walking mechanism, in which β-hydride elimination is a decisive step.
An example of an undesired β-hydride elimination is the β-hydride elimination in the Ziegler-Natta process , which leads to the polymers produced having a reduced chain length. In the case of nickel- and palladium- catalyzed coupling reactions of aryl halides with Grignard reagents , β-hydride elimination can also reduce the yield.
In some cases, β-hydride elimination is the first in a series of steps. This is the case, for example, in the synthesis of RuHCl (CO) (PPh 3 ) 3 from ruthenium (III) chloride , triphenylphosphine and 2-methoxyethanol . There, the alcoholate formed as an intermediate reacts with β-hydride elimination and thereby forms the hydride ligand and the π-bonded aldehyde , which is later converted into the carbonyl ligand .
Avoid β-hydride elimination
There are several strategies to avoid β-hydride elimination. The most common is to use alkyl ligands such as methyl and neo- pentyl ligands that do not have a β-hydrogen atom. β-hydride elimination is also inhibited if a sterically strained alkene would result. This is illustrated , for example, by the stability of metal complexes with norbornyl ligands .
Bulky alkyl ligands, such as tert -butyl or trimethylsilyl ligands, can prevent the β-H atom from attaining a coplanar configuration with the metal and the α and β atoms, which is necessary for the concerted mechanism of β-H elimination . If the metal center does not have a free coordination point, for example because the complex already fulfills the 18-electron rule, β-hydride elimination is also not possible.
In some cases, other ligands can enforce geometries that prevent β-H elimination. Diphosphine ligands, in which the two phosphorus atoms are spatially fixed in the trans position, have this ability . This can be achieved, for example, in that the phosphorus atoms are embedded in a ferrocene unit, which rigidly defines the distance between the coordinating atoms in the complex. Examples are the nickel and palladium complexes of 1,1'-diphosphinoferrocenes, which are structured in such a way that the metal atom is coordinated by two phosphorus atoms in the trans position. Since both metals form square planar complexes, no free binding site can be formed in the cis position to the alkyl ligand, which prevents β-H elimination.
Individual evidence
- ↑ Christoph Elschenbroich: Organometallchemie, 5th, revised edition 2005, Wiley-VCH Weinheim, page 26. ISBN 3-519-53501-7 .
- ↑ Barton K. Bower, Howard G. Tennent "Transition metal bicyclo [2.2.1] hept-1-yls" Journal of American Chemical Society 1972, Volume 94, pp 2512-2514. doi : 10.1021 / ja00762a056