Halogen aromatics
Halogen aromatics (also halogen arenes or aryl halides ) are derived from the group of aromatics or arenes in which one or more hydrogen atoms have been replaced by elements of main group 7 ( halogens ) - fluorine , chlorine , bromine and iodine - of the periodic table . Compounds of the radioactive element astatine have hardly been researched due to the short half-life of astatine.
Aromatic halogen compounds are sometimes highly toxic and are or have been used as insecticides or fungicides . Many of them are now banned.
Selected examples
- Chlorobenzene , an important solvent for oils, fats, resins, etc.,
- 2-chlorophenol , a disinfectant,
- CS gas , a tear gas,
- DDT , an insecticide banned in most western industrialized countries since the 1970s,
- Decabromodiphenylether and Tetrabromobisphenol A are flame retardants ,
- Eosin , a red dye obtained from coal tar ,
- Merbromin (INN) a dye similar to eosin that was marketed in Germany as an antiseptic (trade name Mercuchrom ) before 2003 .
Trunk connections
| Structural formula |

|

|

|

|
| Surname | Fluorobenzene | Chlorobenzene | Bromobenzene | Iodobenzene |
| Melting point | −42 ° C | −45 ° C | −31 ° C | −29 ° C |
| boiling point | 85 ° C | 132 ° C | 156 ° C | 188 ° C |
| Dome model |

|

|

|

|
The simplest compounds are the benzenes substituted with a halogen atom ; they therefore form the parent compounds of halogen aromatic compounds.
presentation
Chlorobenzene and bromobenzene can be prepared by halogenation using chlorine or bromine in the presence of a Lewis acid .
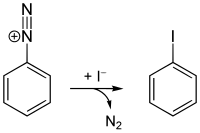
Starting from aniline, all four halogenated benzenes can be prepared by means of diazotization . Chlorobenzene, bromobenzene and iodobenzene are accessible via the Sandmeyer reaction ; the Schiemann reaction is used for fluorobenzene .
- Ph-N N + [BF 4 ] - Ph-F + N 2 + BF 3
properties
Physical Properties
The monohalobenzenes are colorless liquids. The boiling points rise with increasing molar mass. These differences are not as pronounced for the melting points. From fluorine (−42 ° C) to chlorobenzene (−45 ° C) the value even drops, while it then rises moderately towards bromine (−31 ° C) and iodine (−29 ° C).
Chemical properties
A characteristic reaction of halogen aromatic compounds is nucleophilic aromatic substitution . This reaction usually takes place according to the addition-elimination mechanism . Halogen aromatics are seldom subject to nucleophilic substitutions, except in high-energy industrial processes.
Halogen aromatics react with magnesium metal to form Grignard compounds , which are to be regarded as "Ar - " reagents.
Halogen aromatic compounds with two halogen substituents
Substituting benzene with two halogens results in a total of ten combinations. Within each combination there are ortho , meta , and para isomers.
| Fluorobenzene (–F) | Chlorobenzene (–Cl) | Bromobenzene (–Br) | Iodobenzene (–I) | |
| Fluorobenzene (–F) |
Difluorobenzenes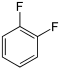  |
Fluorochlorobenzenes | Fluorobromobenzenes | Fluoroiodobenzenes |
| Chlorobenzene (–Cl) |
Dichlorobenzenes 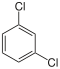 |
Chlorobromobenzenes | Chloroiodobenzenes | |
| Bromobenzene (–Br) |
Dibromobenzenes 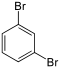 |
Bromiodobenzenes | ||
| Iodobenzene (–I) |
Diiodobenzenes  |
Halogen aromatics with other functional groups
Also known parent aromatic compounds such as phenol, aniline, anisole, toluene, nitrobenzene, benzyl alcohol, benzaldehyde, benzoic acid, etc., there are halogen-substituted derivatives . The name of the root connection does not change. The halogen substituent is placed in front of it as a syllable ( prefix ). Within each group there are ortho , meta , and para isomers.
The −I effect of the halogen substituent leads to a higher acidity of the halophenols and halobenzoic acids compared to phenol or benzoic acid. The pK s values are therefore correspondingly lower (phenol: 9.99 / halogenated phenols: 9.06 - benzoic acid: 4.20 / halobenzoic: 3.58).
Halogenated heteroaromatics
The halogenated heteroaromatic compounds also belong to the group of halogen aromatic compounds, e.g. B. 2-chloropyridine , 2,6-dibromopyridine , 2,4,6-trichlorotriazine , 2,4,6-trifluorotriazine or the insecticide chlorpyrifos .
Individual evidence
- ↑ a b Entry on fluorobenzene in the GESTIS substance database of the IFA , accessed on December 26, 2019(JavaScript required) .
- ↑ a b Entry on chlorobenzene in the GESTIS substance database of the IFA , accessed on December 26, 2019(JavaScript required) .
- ↑ a b Entry on bromobenzene in the GESTIS substance database of the IFA , accessed on December 26, 2019(JavaScript required) .
- ↑ a b iodobenzene data sheet from Sigma-Aldrich , accessed on December 26, 2019 ( PDF ).
- ↑ a b CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 .
- ↑ a b The values are average values of all the pK s values of the derivatives in question and are used here for illustration only.
literature
- Allinger , Cava , de Jongh , Johnson , Lebel , Stevens : Organic Chemistry , 1st Edition, Walter de Gruyter, Berlin 1980, ISBN 3-11-004594-X , pp. 19, 548-556, 602-669.
- Streitwieser / Heathcock : Organic Chemistry , 1st Edition, Verlag Chemie, Weinheim 1980, ISBN 3-527-25810-8 , pp. 692-695, 1053-1064.
- Beyer / Walter : Textbook of Organic Chemistry , 19th edition, S. Hirzel Verlag, Stuttgart 1981, ISBN 3-7776-0356-2 , pp. 445-453.
- Morrison / Boyd : Textbook of Organic Chemistry , 3rd Edition, Verlag Chemie, Weinheim 1986, ISBN 3-527-26067-6 , pp. 700-702, 1113.
- K. Peter C. Vollhardt , Neil E. Schore : Organic Chemistry , 4th Edition, Wiley-VCH, Weinheim 2005, ISBN 978-3-527-31380-8 , pp. 823-824, 1170.