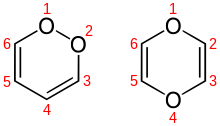
1,4-dioxin
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||
| General | ||||||||||
| Surname | 1,4-dioxin | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 4 H 4 O 2 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 84.07 g mol −1 | |||||||||
| Physical state |
liquid |
|||||||||
| boiling point |
75 ° C |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
1,4-Dioxin , also p-dioxin , is an oxygen-containing heterocyclic organic compound, which can be understood formally as a closed double ether of the dihydric alcohol ethenediol (HO – CH = CH – OH) or as an oxygen heterocycle of cyclohexadiene: 1, 4-dioxa-2,5-cyclohexadiene. In addition to 1,4-dioxin, there is the isomer 1,2-dioxin ( o -dioxin ).
The toxic, carcinogenic and long-lasting polychlorinated dibenzodioxins and furans are derived from the structure of dioxin and the chemically related furan . The term dioxin is widely used outside experts for this class, which includes the so-called Seveso 2,3,7,8-Tetrachlorodibenzodioxin (TCDD) is one that the chemical accident in Seveso , the Seveso disaster was released.
Extraction and presentation
1,4-Dioxin can be prepared by epoxidizing the double bond of the product of the Diels-Alder reaction of furan and maleic anhydride and cleaving it to maleic anhydride and 1,4-dioxin by a retro-Diels-Alder reaction .
Individual evidence
- ↑ CRC Handbook of Data on Organic Compounds, 2nd Edition, Weast, RC and Grasselli, JG, ed (s)., CRC Press, Inc., Boca Raton, FL, 1989, 1.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ R. Alan Aitken, JIG Cadogan, Ian Gosneya: Effect of ring strain on the formation and pyrolysis of some Diels-Alder Adducts of 2-sulfolenes (2,3-dihydrothiophenes 1,1-dioxide) and maleic anhydride with 1.3 -services and products derived therefrom . In: J. Chem. Soc., Perkin Trans. 1 . 1994, pp. 927-931. doi : 10.1039 / p19940000927 .