1,7-dihydroxyanthraquinone
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
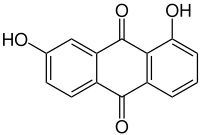
|
||||||||||
| General | ||||||||||
| Surname | 1,7-dihydroxyanthraquinone | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 14 H 8 O 4 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 240.21 g mol −1 | |||||||||
| Melting point |
292 ° C |
|||||||||
| solubility |
|
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
1,7-Dihydroxyanthraquinone , also known under the name m -dihydroxyanthraquinone, is an organic compound from the group of anthraquinones , more precisely the dihydroxyanthraquinones .
presentation
The 1,7-dihydroxyanthraquinone can be prepared from the product ( 3 ) of a Diels-Alder reaction , a diene ( 1 ) and a derivative of juglone ( 2 ) in benzene . Adding concentrated hydrochloric acid in chloroform followed by ethanol gives a yield of approximately 75-80%.

OAc = acetoxy group
1,7-Dihydroxyanthraquinone can also be produced by reacting a benzophenone derivative in a molten salt .
Individual evidence
- ↑ a b Brian Beagley, Anthony DM Curtis, Robin G. Pritchard, Richard J. Stoodley: Highly regio- and stereo-selective Diels-Alder reactions of 5- (2 ', 3', 4 ', 6'-tetra-O -acetyl-β-D-glucopyranosyloxy) -1,4-naphthoquinone. In: J. Chem. Soc., Chem. Commun. 1991, pp. 119-121, doi : 10.1039 / C39910000119 .
- ↑ a b 2nd chlorine dim . In: Ian Heilbron (Ed.): Dictionary of organic Compounds . tape 2 . Eyre & Spottiswoode, Ltd, London 1965, ISBN 0-413-60700-3 , pp. 1050 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ Andre Wahl, FW Atack: The Manufacture Of Organic Dyestuffs . G. Bell and Sons, Limited. 1919. Retrieved June 26, 2017.
- ↑ Vanessa Gonnot, Cyril Antheaume, Marc Nicolas, Charles Mioskowski, Rachid Baati: Highly Selective Three-Step Synthesis of Rhein in Chloroaluminate Molten Salt: Preclusion of the Hayashi Rearrangement. In: European Journal of Organic Chemistry . , 2009, pp. 6205-6210, doi : 10.1002 / ejoc.200900960 .
