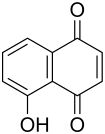
Juglone
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Juglone | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 10 H 6 O 3 | ||||||||||||||||||
| Brief description |
yellow-brown, odorless, crystalline powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 174.16 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
155 ° C |
||||||||||||||||||
| solubility |
heavy in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Juglone is the simplest naturally occurring dye . It is derived from 1,4-naphthoquinone and belongs to the group of carbonyl dyes .
Extraction and presentation
The reaction of 1,5-dihydroxynaphthalene with peracetic acid in excess yields a mixture of juglone and 2-hydroxy-1,4-naphthoquinone .
Occurrence and characteristics
Juglone can be isolated from walnut shells and also produced synthetically. Precursors of juglone are found in the leaves and can get into the soil when they decay, which hinders the formation of roots in other plants. In this way, competitors for water and nutrients grow sparsely under walnut trees. Juglone has antibacterial and fungitoxic action and acts bleeding breastfeeding ( antihämorrhagisch ). Animal studies have shown that juglone is also mutagenic .
Juglone is isomeric to 2-hydroxy-1,4-naphthoquinone (Lawson), the dye found in henna leaves .
See also
literature
- Paul Karrer: Textbook of Organic Chemistry . 10th edition. Thieme Verlag, Stuttgart 1948.
- Dietrich Frohne, Uwe Jensen: Systematics of the Plant Kingdom , 5th edition, Wissenschaftliche Verlagsgesellschaft mbH, Stuttgart 1998.
Individual evidence
- ↑ Juglon data sheet (PDF) from Carl Roth , accessed on December 14, 2010.
- ^ The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals , 14th Edition, 2006, p. 911, ISBN 978-0-911910-00-1 .
- ↑ a b c Entry on 5-hydroxy-1,4-naphthoquinone in the GESTIS substance database of the IFA , accessed on February 7, 2017(JavaScript required) .
- ^ Heinrich Zollinger: Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments . 3. Edition. WILEY-VCH Verlag, Weinheim 2003, ISBN 3-906390-23-3 , p. 347 ( limited preview in Google Book search).
- ^ Christoph Grundmann : A New Synthesis of Juglone . In: Synthesis . tape 1977 , no. 9 , September 1977, p. 644-645 , doi : 10.1055 / s-1977-24517 ( PDF ).
- ^ Albert Gossauer: Structure and reactivity of biomolecules , Verlag Helvetica Chimica Acta, Zurich, 2006, p. 277, ISBN 978-3-906390-29-1 .
- ↑ Botany-online: Influence of individual species on others; Competition, coexistence ( Memento from February 13, 2013 in the Internet Archive )



