1,2-octanediol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
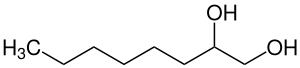
|
||||||||||||||||
| Simplified structural formula without stereochemistry | ||||||||||||||||
| General | ||||||||||||||||
| Surname | 1,2-octanediol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 8 H 18 O 2 | |||||||||||||||
| Brief description |
white solid with a characteristic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 146.23 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
0.93 g cm −3 |
|||||||||||||||
| Melting point |
30-35 ° C |
|||||||||||||||
| boiling point |
267 ° C |
|||||||||||||||
| solubility |
slightly soluble in water (7.5 g l −1 at ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
1,2-Octanediol is a chemical compound from the group of alkanediols that occurs in two isomeric forms.
Extraction and presentation
1,2-Octanediol can be obtained by oxidizing 1-octene .
properties
1,2-Octanediol is a flammable, hardly inflammable, white solid with a characteristic odor, which is sparingly soluble in water.
use
1,2-Octanediol can be used as an organic modifier to improve the HPLC separation of organic acids and bases. It can also be used to make halohydrin palmitates. It is useful for clinically treating head lice infestations . It is also used in coating materials, sludges, paper mills and water cycle systems for effective preservation against bacteria and fungi. It is used as an emollient , humectant and wetting agent in cosmetics and skin care products.
literature
- Lu S. Chen, Simone M. Mantovani et al. a .: 1,2-Octanediol deracemization by stereoinversion using whole cells. In: Journal of Molecular Catalysis B: Enzymatic. 54, 2008, p. 50, doi : 10.1016 / j.molcatb.2007.11.022 .
Individual evidence
- ↑ a b c d e f g h i Entry on 1,2-octanediol in the GESTIS substance database of the IFA , accessed on December 2, 2018(JavaScript required) .
- ↑ Xu-Wei Wu, Bin-Dong Li. Preparation of high purity 1,2-diols by catalytic oxidation of linear terminal alkenes with H 2 O 2 in the presence of carboxylic acids under solvent-free conditions Chinese Chemical Letters , 2014,25 (03): pp. 459-462.
- ↑ Google Patents: US7385092B2 - Process for preparing alkanediols and alkanetriols having a vicinal diol group - Google Patents , accessed December 2, 2018.
- ↑ Data sheet 1,2-octanediol, 98% from Sigma-Aldrich , accessed on December 2, 2018 ( PDF ).
- ↑ Data sheet 1,2-octanediol, 97% from AlfaAesar, accessed on December 2, 2018 ( PDF )(JavaScript required) .
- ↑ I. Jiménez, G. Montalvo, E. Rodenas: Study of 1,2-Octanediol as Cosurfactant of Sodium Dodecyl Sulfate. In: Langmuir . 16, 2000, p. 8604, doi : 10.1021 / la0003589 .
