1-bromo-2-methylpropane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
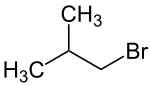
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 1-bromo-2-methylpropane | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 9 Br | |||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 137.02 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.27 g cm −3 |
|||||||||||||||
| Melting point |
−119 ° C |
|||||||||||||||
| boiling point |
92 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.4350 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
1-Bromo-2-methylpropane (also isobutyl bromide ) is a chemical compound from the group of saturated bromohydrocarbons and one of the isomeric butyl bromides .
Extraction and presentation
1-Bromo-2-methylpropane can be prepared by reacting isobutyl alcohol with phosphorus (III) bromide . It can also be obtained in small quantities by bromination of 2-methylpropane ( isobutane ), whereby mainly 2-bromo-2-methylpropane is formed.
properties
1-Bromo-2-methylpropane is a volatile, colorless liquid that is very sparingly soluble in water.
safety instructions
The vapors of 1-bromo-2-methylpropane can form an explosive mixture with air ( flash point 7 ° C).
Individual evidence
- ↑ a b c d e f g h i j k l m Entry on 1-bromo-2-methylpropane in the GESTIS substance database of the IFA , accessed on June 10, 2017(JavaScript required) .
- ↑ a b Data sheet 1-Bromo-2-methylpropane, 98 +% from AlfaAesar, accessed on July 20, 2013 ( PDF )(JavaScript required) .
- ↑ Joachim Buddrus: Fundamentals of organic chemistry . Walter de Gruyter, 2011, ISBN 3-11-024894-8 , p. 315 ( limited preview in Google Book search).
- ^ Kurt Peter C. Vollhardt, Neil Eric Schore: Organic Chemistry . John Wiley & Sons, 2011, ISBN 3-527-32754-1 , pp. 129 ( limited preview in Google Book search).
