2-fluoronaphthalene
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 2-fluoronaphthalene | |||||||||||||||
| other names |
β-fluoronaphthalene |
|||||||||||||||
| Molecular formula | C 10 H 7 F | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 146.16 g mol −1 | |||||||||||||||
| Melting point |
58 ° C |
|||||||||||||||
| boiling point |
212 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
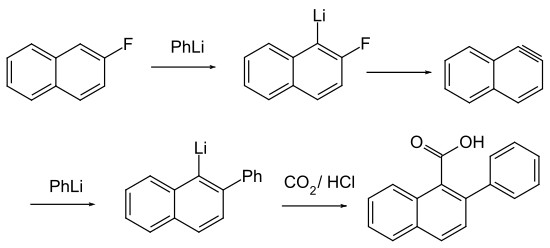
2-fluoronaphthalene ( β-fluoronaphthalene ) is a chemical compound of fluorine from the group of naphthalene derivatives and is a fluoroaromatic compound. 2-fluoronaphthalene is isomeric to 1-fluoronaphthalene .
Extraction and presentation
Aryl fluorides are prepared from diazonium fluoroborates in a Schiemann reaction .
Fluorination with N -fluorobis (phenylsulfonyl) amine is also possible.
properties
It is a white solid.
Usage and reactions
The fluorinated aromatics are used for coupling reactions of aryls.
literature
- Nobuo Nakata: Attempts on the question of the naphthalene model . In: Reports of the German Chemical Society . tape 64 , no. 8 , 1931, p. 2059-2069 , doi : 10.1002 / cber.19310640829 .
- Günther Schiemann, Werner Gueffroy, Wolfgang Winkelmüller: Fluorine compounds of naphthalene. In: Liebig's annals . Volume 487, No. 1, 1931, pp. 270-287, doi: 10.1002 / jlac.19314870118 .
- FW McLafferty: Mass Spectrometric Analysis. Aromatic Halogenated Compounds . In: Analytical Chemistry . tape 34 , no. 1 , 1962, pp. 16-25 , doi : 10.1021 / ac60181a004 .
- Haget Bouillard, Y. et al. Acta Cryst. 1972, 28, 3400. in AI Kitaigorodsk: Mixed Crystals , 33, Springer Series in Solid-State Sciences, 377. ISBN 9783642816727 .
- David Doddrell, Michael Barfield, William Adcock, Mohammad Aurangzeb, David Jordan: 13 C nuclear magnetic resonance studies of some fluorinated and trifluoromethylated aromatic compounds. Studies on 13 C– 19 F coupling constants . In: J. Chem. Soc. , Perkin Trans. 2 . tape 0 , no. 4 , 1976, p. 402-412 , doi : 10.1039 / P29760000402 .
- Jason R. Loader, Stefano Libri, Anthony JHM Meijer, Robin N. Perutz, Lee Brammer: Highly fluorinated naphthalenes and bifurcated C – H ⋯ F – C hydrogen bonding . In: CrystEngComm . tape 16 , no. 41 , 2014, p. 9711-9720 , doi : 10.1039 / c4ce01322k .
Individual evidence
- ^ A b c d William M. Haynes: CRC Handbook of Chemistry and Physics, 93rd edition . CRC Press, 2012, ISBN 978-1-4398-8049-4 , pp. 276 ( limited preview in Google Book search).
- ↑ Data sheet 2-Fluoronaphthalene solution, 2000 μg / mL in methylene chloride from Sigma-Aldrich , accessed on December 3, 2017 ( PDF ).
- ↑ There is not yet a harmonized classification for this substance . A labeling of 2-fluoronaphthalene in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on December 3, 2017, is reproduced from a self-classification by the distributor .
- ↑ H. Meislich, H. Nechamkin, J. Sharefkin: Organic Chemistry. Schaum, Mc Graw Hill Book Company 1980, ISBN 007-092028-2 , 404.
- ↑ Jerry March: Advanced Organic Chemistry , pp. 1929-1985, 602, ISBN 0-471-85472-7 .
- ↑ GI Borodkin, IR Elanov, and VG Shubin: Fluorination of Bi- and Polycyclic Aromatic Hydrocarbons with N-Fluorobis (phenylsulfonyl) amine in the Absence of Solvent . In: Zhurnal Organicheskoi Khimii , 2010, Vol. 46, No. 9, pp. 1318-1323.
- ^ R. Huisgen et al .: The reaction of aromatic fluorine compounds with phenyllithium; a contribution to the chemistry of rearrangements in nucleophilic aromatic substitutions , 1955, doi: 10.1002 / jlac.19555940204 .