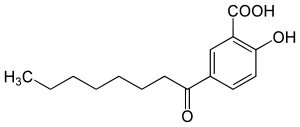
2-hydroxy-5-octanoylbenzoic acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2-hydroxy-5-octanoylbenzoic acid | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 15 H 20 O 4 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 264.32 g · mol -1 | ||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
The 2-hydroxy-5-octanoylbenzoesäure , also β-Lipohydroxysäure ( LHA ) is a by a lipophilic chain of eight carbon atoms substituted salicylic acid . This belongs to the group of beta-hydroxy acids (BHA) and is a natural substance that occurs in various plants (e.g. willow bark). LHA is used as a cosmetic product as an active peeling ingredient.
history
LHA was developed by L'Oréal Recherche in the late 1980s . Various synonyms for the substance are given in the literature.
properties
LHA is a derivative of salicylic acid in which an octanoyl radical is linked to the 5 'position of the salicylic acid. It is insoluble in water.
pharmacology
Because of its hydrophobic character, LHA does not penetrate the skin as deeply as salicylic acid. Only about 6% reach deeper layers of the skin than the stratum corneum , the outermost layer of the epidermis. There it causes targeted desquamation at the corneocyte level by breaking the desmosomes (corneosomes) in between. Since the break occurs at the interface with the next lower layer, the stratum disjunctum , these breaks are relatively exact. In addition to the peeling effect, LHA is said to stimulate cell division in the upper layer of the skin.
LHA also has a protective effect against dermatological damage caused by UV light . It can render reactive oxygen particles ( singlet oxygen 1 O 2 , hyperoxide anions O 2 - ) harmless that are formed by UV light . In addition, the acid could also have an antibacterial as well as an antimicrobial effect against Malassezia furfur .
Finally, LHA shows good skin tolerance.
Individual evidence
- ↑ a b D. Saint-Léger et al .: The use of hydroxy acids on the skin: characteristics of C 8 lipohydroxy acid . In: Journal of Cosmetic Dermatology , 2007 , 6 (1) ; 59-65; PMID 17348998 ; doi : 10.1111 / j.1473-2165.2007.00296.x .
- ↑ There is not yet a harmonized classification for this substance . A labeling of [No public or meaningful name is available] in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), retrieved on January 20, 2020, is reproduced from a self-classification by the distributor .
- ^ GE Piérard and A. Rougier: Nudging acne by topical beta-lipohydroxy acid (LHA), a new comedolytic agent . In: European Journal of Dermatology , 2002 , 12 (4) , XLVII-XLVIII; PMID 12120612 .

