Three-center bond
The three- center bond , the simplest multi-center bond , is a special type of atomic bond ( covalent bond ) in which not two atoms, but three atoms share an electron pair. According to modern atomic theory ( orbital theory ), three-center bonds are described by the overlapping of three atomic orbitals , whereby three molecular orbitals arise. One of these is always binding and one is antibonding . The third molecular orbital can have weakly binding, weakly antibonding or non-binding character. Prime examples of three-center bonds can be found in the H 3 + ion, in diborane (B 2 H 6 ), in the hydrogen difluoride ion (FHF - ) or in the nitrite ion (NO 2 - ). In connection with three- or multi-center bonds , one speaks of a delocalization of the bonding electrons . Two-center bonds - the normal covalent bonds - and free electron pairs, on the other hand, are referred to as localized .
Open and closed three-center bonds
A distinction is made between open three-center bonds - as found in the FHF - ion - and closed three-center bonds such as that found in the H 3 + ion. In the first case, two of the three atoms show no significant orbital overlap (here the two fluorine atoms ), in the second case there is a paired overlap of all three atomic orbitals - a ring is formed. As the angle increases, an open three-center bond can change into a closed one. While open three-center bonds can contain up to four electrons - up to two of them are found in a nonbonding orbital - a closed three-center bond is only stable if at most two electrons are accommodated in the molecular orbitals.
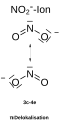
3-center-2-electron-bond and 3-center-4-electron-bond
The terms 3-center-2-electron bond (3c-2e) or 3-center-4-electron bond (3c-4e) are often found in the literature . These terms indicate how many electrons for a given molecule have been accommodated in the three molecular orbitals that result from the three-center interaction. However, since only one of the three molecular orbitals can be regarded as significantly binding and a maximum of two electrons can be accommodated in this orbital, only the term 3-center-2-electron bond is used consistently. With four electrons, the non-bonding orbital is also occupied twice. Since this orbital only has significant contributions (coefficients) on the two outer atoms, these electrons "resemble" lone pairs of electrons on these atoms. The bonding relationships in these 3-center-4-electron bonds can accordingly be formulated using two mesomeric boundary structures. There is a free electron pair on each of the two outer atoms, which points in the direction of the central atom, while the other forms a bond to the central atom (bond / no-bond boundary structure) (see HF 2 - ion).
Symbols
At least two mesomeric boundary structures are required to adequately represent a three-center bond with Lewis formulas . In addition, special symbols have become established, but they are not used and accepted throughout, as they can be confused with the skeletal formula .