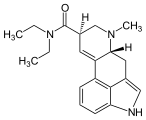
ALD-52
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||
| General | ||||||||||
| Surname | ALD-52 | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 22 H 27 N 3 O 2 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| Drug information | ||||||||||
| Mechanism of action | ||||||||||
| properties | ||||||||||
| Molar mass | 365.47 g mol −1 | |||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
ALD-52 (1- acetyl - D - lysergic acid diethylamide ) is a psychedelic acting psychotropic substance and research chemical . ALD-52 is a derivative of lysergic acid , which occurs naturally as an ergot alkaloid , as well as an analogue of LSD and an acyl homologue of 1P-LSD and 1B-LSD . ALD-52 was the subject of ergoline and psychedelics research in the 1960s and shows the same effects as LSD.
chemistry
Chemically, ALD-52 belongs to the Ergoline structural class . The tetracyclic ergoline is characteristic of the chemical structure of ergot alkaloids . In contrast to LSD, ALD-52 has an additional N 1 - acetyl group . Chemical modifications in the N 1 position are among the most frequently carried out changes to the ergoline system, since the indole nitrogen is easily accessible for alkylations , acylations , Mannich reactions and Michael additions .
literature
- H. Isbell, EJ Miner, CR Logan: Relationships of psychotomimetic to anti-serotonin potencies of congeners of lysergic acid diethylamide (LSD-25). In: Psychopharmacologia. Volume 1, 1959, pp. 20-28. PMID 14405872 .
- B. Berde, W. Doepfner, A. Cerletti: About the duration of action of some serotonin antagonists . In: Helv. Physiol. pharmacol. Acta. 18, 1960, pp. 537-544.
- AL Halberstadt, M. Chatha u. a .: Pharmacological and biotransformation studies of 1-acyl-substituted derivatives of d-lysergic acid diethylamide (LSD). In: Neuropharmacology. November 2019, doi : 10.1016 / j.neuropharm.2019.107856 , PMID 31756337 .
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Jacob, P; Shulgin, AT. Structure-activity relationships of the classic hallucinogens and their analogs. In Hallucinogens: An update. NIDA Research Monograph 146; Lin, GC; Glennon, RA, Eds., US Department of Health and Human Services, National Institute of Health, US Government Printing Office, Washington, DC, 0000; pp 74-91.
- ↑ HA Abramson: Lysergic Acid Diethylamide (LSD-25): XXXI. Comparison by Questionnaire of Psychotomimetic Activity of Congeners on Normal Subjects and Drug Addicts. In: The British Journal of Psychiatry. 106, 1960, p. 1120, doi: 10.1192 / bjp.106.444.1120 PMID 13681136 .
- ↑ Abram Hoffer, Humphry Osmond: The hallucinogens . Academic Press, 1967 ( limited preview in Google Book Search).
- ↑ Petr Bulej, Ladislav Cvak: Ergot: The Genus Claviceps. Medicinal and Aromatic Plants - Industrial Profiles . Ed .: Vladimir Kren, Ladislav Cvak. CRC Press, 2004, ISBN 0-203-30419-5 , Chemical modifications of ergot alkaloids, pp. 202-230 .