Acid-base titration
Acid-base titrations are dimensional analytical methods for determining the molar concentration of acids or bases in a pure substance solution . In the case of mixtures of substances, this determines the acid capacity or the base capacity (see also buffer capacity ).
Instead of the generic term acid-base titration, the determination of the molar concentration of an acid with the help of a base is also called alkalimetry . Similarly, the determination of the molar concentration of a base with the aid of an acid is also referred to as acidimetry .
The determination is made by the dosed addition ( titration ) of a suitable standard solution from a burette . For alkalimetry, the basic (alkaline) solution of a strong base (often 0.1 molar sodium hydroxide solution ) is used as the standard solution , for acidimetry the acidic solution of a strong acid (often 0.1 molar hydrochloric acid ). In the course of the titration, the pH of the sample solution changes due to the neutralization reaction taking place in the direction of the neutral point , since H 3 O + or OH - are converted to H 2 O. The end point of the titration, which, depending on the type of acid or base to be determined, is characterized by a more or less strong change in the pH value, is known as the equivalence point . The equivalence point can be indicated by the color change of a suitable indicator if the pH value at the equivalence point changes significantly or even abruptly during the titration. If this is not the case, the equivalence point can also be determined by using a pH electrode and graphically evaluating the titration curve obtained. The pH value at the equivalence point depends on the anions (and cations) formed during the titration. If strong acids (e.g. HCl, HNO 3 , H 2 SO 4 ) or bases (e.g. NaOH, KOH) have been titrated, then only the anions of strong acids are present at the equivalence point and the equivalence point is pH = 7. If weak acids have been titrated, other anions are present at the equivalence point (e.g. phosphate, carbonate, acetate) and the equivalence points are in higher pH ranges. If a color indicator is used for such titrations, the appropriate indicator must be selected to display the equivalence point, which only shows its color change at higher pH values. If multi-proton weak acids (e.g. H 3 PO 4 ) are titrated, several equivalence points can be expected at different pH values.
Course of titration curves
| Alkalimetry |

|
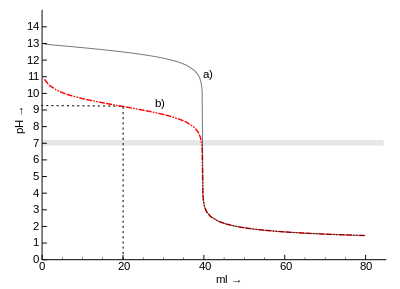
| Titration curves of a) hydrochloric acid b) acetic acid against sodium hydroxide solution. Half-equivalence point of acetic acid (dashed, at 20 ml): pH = p K s = 4.75. |
| Acidimetry |
|
| Titration curves of a) sodium hydroxide solution b) ammonia versus hydrochloric acid. Half-equivalence point of ammonia (dashed, at 20 ml): pH = p K s = 9.25 |
Titration curves of aqueous solutions of very strong acids and very strong bases all have a similar course. During the reaction, oxonium and hydroxide are converted quantitatively into water:
They are the only proton donors or proton acceptors in such aqueous solutions. The cause is the leveling of very strong acids and bases. In the case of hydrochloric acid , hydrogen chloride is the very strong acid that has been (formally or real) hydrolyzed:
In the case of caustic soda , the very strong base is sodium hydroxide , which was completely hydrolyzed when reacted with water:
Measured variables are the volume of the sample solution, the added volume of standard solution and the respective pH value of the solution. In the acidic range, the pH value of the sample solution increases
- certainly.
In the basic range, the pH value is above
- and with through
- certainly.
The autoprotolysis of water
is negligibly low in almost all areas, but determines the pH value at the equivalence point with pH = 7 at 25 ° C.
Titration curves of aqueous solutions of medium-strength acids and medium-strength bases show a different course up to the equivalence point, since the dissolved acids or bases are not completely hydrolyzed. Besides the implementation
takes place in alkalimetry
or in acidimetry
The particles designated as acid and base in the last two reactions are the respective conjugated acid-base pairs , in the upper curve it is acetic acid and the acetate ions , in the lower curve the ammonium ions and ammonia . If the concentration and volume of the sample solution and the titrand are known, the course of the titrations can be estimated by calculation. When titrating medium-strength acids or bases, the protolysis of acetic acid or ammonia with water can be neglected (apart from the starting point) and a quantitative conversion of the acid or base to be determined with OH - or H 3 O + can be assumed. The pH value of the respective solution is determined by the existing concentrations of the acid-base pairs and is determined by the Henderson-Hasselbalch equation
described. In the case of the determination of acetic acid, the proton donor is acetic acid itself, with a p K s value of 4.75, and in the case of the determination of ammonia, the ammonium ion, with a p K s value of 9.25. With a 50% conversion, the respective proton donors and acceptors are present in the same concentration and the pH value is equal to the respective p K s value:
This point is sometimes called the half-equivalence point . Around this point, the change in the pH value in the course of the titration is particularly flat because buffer solutions are available. From the equivalence point onwards, the pH curve is only determined by adding the standard solution.
Choice of indicator
The color change of a suitable indicator should be in the area of the equivalence point (almost vertical course of a titration curve).
The envelope of pH indicators is generally two pH units wide. There is also an acid-base reaction with the indicators:
- (see e.g. methyl red )
The indicators follow the Henderson-Hasselbalch equation and one indicator also has a p K s value. Because of their low concentration, however, the course of titration curves remains largely unaffected by indicators.
Since sodium carbonate (freedom from water by drying in an oven at 200 ° C) is often used to produce a very precise Urtiter solution for acid measurement solutions, methyl orange is a very important color indicator for the exact setting of acids.
The indicator bromothymol blue is suitable for titrating a strong acid with a strong base such as hydrochloric acid and sodium hydroxide solution, as its color changes at a pH value of around 6.0 to 7.6, which is in the range of the equivalence point. If, on the other hand, the concentration of a medium-strength acid such as acetic acid is to be determined with the aid of sodium hydroxide solution, the indicator phenolphthalein , for example , whose transition range from colorless to red-purple is in the pH range from 8.2 to 10, is used. Methyl red , with a transition range from pH 4.4 to 6.2, is suitable for the determination of medium-strength bases such as ammonia.
Titration with a pH meter
The end point of the titration can also be determined with the help of a pH meter , i.e. with an electrical measuring device. This measuring method provides a clear result that does not depend on the experience of the person carrying out the work. The pH value of the sample solution depending on the volume of the standard solution added step by step can be displayed and evaluated in a titration curve.
Since polyvalent acids in particular have so-called buffer capacities , in which the pH value remains constant for a relatively long time during the titration and the neutral point can be reached quite suddenly, this behavior can be better observed with a pH meter. There is of course no need to use an indicator.
Automatic titration
- → Main article: Laboratory automation
A further development of the titration with the pH meter means that not only the pH value is recorded electronically by a computer, but also the addition of the titration liquid can be regulated automatically. In addition, the computer connected to the titration apparatus can process the results immediately and e.g. B. convert into a concentration value. The titration can thus be completely automated.
Remarks
- ↑ The terms alkalimetry and acidimetry are used inconsistently in the literature. Occasionally, alkalimetry is understood to mean the determination of the content of a base and acidimetry is understood to mean the determination of the content of an acid. With other methods of titrimetry , however, the titrants used give the name, such as B. in iodometry or manganometry . The above definition of the two terms is therefore uniform and therefore advantageous.
- ↑ Calculated conversions of 40 ml 0.1 molar solutions with 0.1 molar standard solutions.
literature
- G. Jander, KF year, G. Schulze: measure analysis. 16th edition, de Gruyter, Berlin 2003, ISBN 3-11-017098-1 .
Web links
- ^ TL Brown, HE Le May: Chemistry, a textbook for all natural scientists , VCH Verlagsgesellschaft, Weinheim 1988, ISBN 3-527-26241-5 , pp. 522-525.















