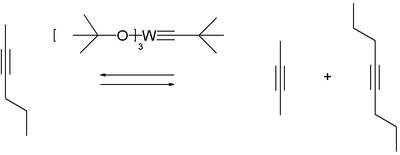Alkyne metathesis
In the alkyne metathesis is an organic reaction in which substituents on a alkinischen be replaced triple bonds. The reaction is closely related to alkene metathesis .
Metal-catalyzed alkyne metathesis was first proposed by Bailey et al. Described in 1968. The working group used a mixture of tungsten and silicon dioxide at temperatures of up to 450 ° C as a catalyst . In 1974 Mortreux published results that a homogeneous catalyst ( molybdenum hexacarbonyl at 160 ° C) was also able to exchange alkynic triple bonds; a non-symmetrically substituted alkyne reacted in an equilibrium reaction to give the two symmetrically substituted alkynes.
history
The Mortreux system consists of the catalyst molybdenum hexacarbonyl Mo (CO) 6 and a resorcinol - cocatalyst . In 1975, TJ Katz proposed an ethynyl metal and a metallacyclobutadiene as intermediates . In 1981 Richard R. Schrock characterized various catalytically active metallacyclobutadiene complexes .
Tris ( tert -butoxy) (neopentylidine) tungsten (VI) is a Schrock catalyst that is unreactive towards alkenes . ( Fischer carbenes can not be used for alkyne and alkene metathesis reactions .)
The Schrock catalyst is commercially available. It is produced by the first step, tungsten (IV) chloride with lithium dimethylamide to W 2 (NMe 2 ) 6 amidated is. The resulting compound is then reacted with tert -butanol . Finally, a metathesis reaction of the complex with neohexine is carried out to give the end product.
Ring closure by means of alkyne metathesis
Alkyne metathesis is often used to carry out ring-closing reactions. The scent civetone can be represented from a Diin , for example . After ring closure, the triple bond is stereoselectively hydrogenated with hydrogen over a Lindlar catalyst in order to obtain only the Z isomer . (The E isomer could be obtained by reduction with an alkali metal in liquid ammonia and a weak acid such as ethanol.) An important driving force for such reactions is the outgassing of small molecules such as ethyne or 2-butyne .
The same procedure was used in the synthesis of the naturally occurring cyclophane Turrian.
Cross metathesis with nitrile and alkyne
In this cross metathesis, a tungsten nitride is used instead of an alkylidyne tungsten compound . In this way, two nitriles can be coupled to form an alkyne. The nitrogen is transferred to a sacrificial alkyne, so there is no elemental nitrogen :
Web links
Individual evidence
- ↑ Fürstner, A .; Davies, PW: Alkyne metathesis . In: Chemical Communications . No. 18, 2005, pp. 2307-2320. doi : 10.1039 / b419143a .
- ↑ Fürstner, A .; Mathes, C .; Lehmann, CW: Mo [N ( t -Bu) (Ar)] 3 Complexes As Catalyst Precursors: In Situ Activation and Application to Metathesis Reactions of Alkynes and Diynes . In: J. Am. Chem. Soc. . 121, No. 40, 1999, pp. 9453-9454. doi : 10.1021 / ja991340r .
- ^ RR Schrock, DN Clark, J. Sancho, JH Wengrovius, SM Rocklage, SF Pedersen: Tungsten (VI) neopentylidyne complexes. In: Organometallics. 1, 1982, pp. 1645-1651, doi : 10.1021 / om00072a018 .
- ↑ Geyer, AM; Gdula, RK; Wiedner, ES; Johnson, MJA: Catalytic Nitrile-Alkyne Cross-Metathesis . In: J. Am. Chem. Soc. . 129, No. 13, 2007, pp. 3800-3801. doi : 10.1021 / ja0693439 .
- ^ Ritter, S .: Nitrile-Alkyne Cross-Metathesis . In: Chemical & Engineering News , March 26, 2007.