Civetone
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
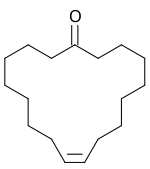
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Civetone | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 17 H 30 O | |||||||||||||||
| Brief description |
colorless crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 250.41 g · mol -1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
0.91 g cm −3 |
|||||||||||||||
| Melting point |
32.5 ° C |
|||||||||||||||
| boiling point |
343 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.4820 (at 37 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Civetone is a chemical compound from the group of cyclic ketones .
Occurrence
The natural substance civetone is a main component of the fragrance civet - the anal secretion of the African civet cat . It is one of the oldest known perfume ingredients. Civetone is closely related to muscone . Both compounds have the structure of a large cyclic molecule. The structure was clarified by Leopold Ružička in 1926.
Extraction and presentation
Zibetone can be obtained synthetically by Dieckmann cyclization ( Dieckmann condensation ) of 9-octadecene-1,18-dicarboxylic acid dialkyl esters or Ti-Claisen condensation .
use
Civetone is widely used as a fragrance in perfumes, soaps, and other household chemicals. To lure wild cats such as jaguars, cheetahs and tigers near an automatic camera trap , field biologists use Calvin Klein's eau de Cologne "Obsession for men" as a lure, which contains synthetic civetone, among other things.
Individual evidence
- ↑ Entry on CIVETONE in the CosIng database of the EU Commission, accessed on February 26, 2020.
- ↑ a b c Cycloheptadeca-9-en-1-one (JECFA evaluation)
- ↑ a b Walter Widmer: Contribution to the synthesis of cibetone , 1942, ETH Zurich, doi: 10.3929 / ethz-a-000092338 .
- ↑ a b Entry on cibetone in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on October 24, 2017.
- ↑ There is not yet a harmonized classification for this substance . A labeling of (Z) -9-cycloheptadecen-1-one in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on October 24, 2017, is reproduced from a self-classification by the distributor .
- ↑ MSDS on chinaperfumer.com (PDF file; 33 kB).
- ↑ freshpatents.com ( Memento of the original from October 19, 2013 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Development of Efficient and Practical Ti-Claisen Condensation and the Related Aldol Reaction (PDF file; 280 kB).
- ↑ https://medium.com/starts-with-a-bang/weekend-diversion-obsession-for-cats-7266391e2861
