Semi-aminals
| Semi-aminals |
|---|

|
| General structure of the hemiaminals with the amino and hydroxyl groups marked in blue , both of which are bonded to the same carbon atom. The radicals R 1 , R 2 , R 3 and R 4 independently represent an aliphatic , cyclic or aromatic radical or a hydrogen atom . |
Half-aminals (synonym: half-aminoacetal ) form a group of substances in organic chemistry that have a hydroxyl group and an amino group , which are bonded to the same carbon atom, as a characteristic structural element . Half-aminals are usually quite unstable (see Erlenmeyer rule ) and tend to form imines or enamines when water is split off . In the presence of dilute acids, this reaction occurs almost immediately. Hemaminals can also be understood as nitrogen analogs of hemiacetals in which the alkoxy group (O-R 1 ) has been replaced by an amino group. In a certain way, hemiaminals can also be viewed as α-amino alcohols .
Aminals (also aminoacetals ) are a group of substances that contain two amino groups attached to a carbon atom as functional groups .
Manufacturing
Reaction mechanism
Aldehydes and ketones ( carbonyl compounds ) react with ammonia , primary or secondary amines in a nucleophilic addition reaction with one another and initially form hemiaminals. The following reaction scheme is intended to clarify the mechanistic details of this reaction:
In the first step, the corresponding carbonyl compound reacts with ammonia (R 3 , R 4 = H), a primary (R 3 = H, R 4 = organyl radical) or secondary amine (R 3 , R 4 = organyl radical). The nitrogen lone pair of electrons nucleophilically attacks the positively polarized carbonyl carbon atom ( 1 ). The addition product arises as the transition state ( 2 ) , which can be understood as either an alcoholate or a quaternary ammonium compound . The last reaction step involves the tautomerization of a hydrogen atom ("protonation"). The alcoholate anion deprotonates the ammonium group and forms a hydroxyl group . Furthermore, the ammonium cation is converted back to a primary, secondary or tertiary amine (depending on R 3 and R 4 ). Finally, the corresponding hemi-aminal is created ( 3 ).
Examples
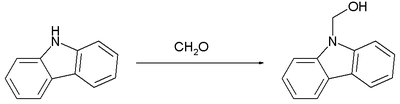
The following reaction shows an example of the formation of a hemiaminal from the secondary amine carbazole and formaldehyde .
Aminals of primary amines are unstable and often cannot be observed directly. This usually requires special conditions.
The formation of hemiaminales is a key reaction in the stereoselective synthesis of saxitoxin :
In this step, the double bond is first oxidized to the intermediate acyloin by osmium (III) chloride, potassium peroxomonosulphate ("oxone") and sodium carbonate. The synthesis of the 3-oxazolines is described in the literature.
Individual evidence
- ↑ K. Peter C. Vollhardt, Neil E. Schore, Katrin-M. Roy, Holger Butenschön: Organic Chemistry . 5th edition. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 2011, p. 855, ISBN 978-3-527-32754-6
- ↑ Milata Viktor, Kada Rudolf, Lokaj Jn: Carbazol -9-yl-methanol. In: Molbank. 2004, 2004, p. M354, doi : 10.3390 / M354 .
- ↑ Reaction in refluxing ethanol with potassium carbonate . Acid catalysis reacts the hemiaminal to the aminal N, N´-biscarbazol-9-yl-methane.
- ↑ Stabilization of Labile Carbonyl Addition Intermediates by a Synthetic Receptor Tetsuo Iwasawa, Richard J. Hooley, Julius Rebek Jr. Science 317, 493 ( 2007 ) doi : 10.1126 / science.1143272 .
- ↑ (+) - Saxitoxin: A First and Second Generation Stereoselective Synthesis James J. Fleming, Matthew D. McReynolds, and J. Du Bois J. Am. Chem. Soc. , 129 (32), 9964-9975, 2007 . doi : 10.1021 / ja071501o .
- ↑ Maya Weber, Jürgen Jakob and Jürgen Martens : Synthesis and reactivity of 3-oxazolines , Liebigs Annalen der Chemie 1992 , 1-6.