Ammonium chromate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
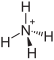 
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ammonium chromate | |||||||||||||||
| other names |
Ammonium monochromate |
|||||||||||||||
| Molecular formula | (NH 4 ) 2 CrO 4 | |||||||||||||||
| Brief description |
yellow-orange, odorless crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 152.07 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.8 g cm −3 |
|||||||||||||||
| Melting point |
185 ° C (decomposition) |
|||||||||||||||
| solubility |
good in water (340 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
Switzerland: 5 μg m −3 (calculated as chromium) |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Ammonium chromate is the ammonium salt of chromic acid with the empirical formula (NH 4 ) 2 CrO 4 .
properties
Ammonium chromate is in the form of yellow, water-soluble crystals, which decompose explosively at 185 ° C to form ammonia , nitrogen , chromium (III) oxide and water . Furthermore, due to its oxidizing properties, ammonium chromate can react dangerously with reducing agents or combustible substances. Aqueous solutions react neutrally .
Manufacturing
Dissolution of chromium (VI) oxide in an ammonia solution :
use
Ammonium chromate is used in the manufacture of iron-chromium oxide catalysts. In addition, ammonium chromate can be used to represent lead chromate .
safety instructions
Ammonium chromate is classified as carcinogenic, especially if it is absorbed via the respiratory tract. Skin contact can lead to sensitization. Appropriate protective clothing must therefore be worn when handling.
Legal notices
Ammonium chromate is subject to the Chemicals Prohibition Ordinance and the Water Resources Act . In industrial quantities, it is also subject to the Hazardous Incident Ordinance .
Ammonium chromate is ecotoxicologically classified as WGK 3 (highly hazardous to water).
Individual evidence
- ↑ a b c d e f g h i Entry on ammonium chromate in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Not explicitly listed in Regulation (EC) No. 1272/2008 (CLP) , but with the indicated labeling it falls under the group entry Chromium (VI) compounds, with the exception of barium chromate and of compounds specified elsewhere in this Annex in the Classification and Labeling inventory of the European Chemicals Agency (ECHA), accessed on August 14, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for chromium (VI) compounds ), accessed on November 9, 2015.
- ↑ Patent DE3819437A1 .

![{\ mathrm {\ \! \ {\ Biggr]} _ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/574adab1409cb81da6c38bb738ad111e61bbb2d9)
![{\ mathrm {\ \! \ {\ Biggr]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/c9fd9553a0b623f5f669641be907d9186e35b739)




