Ammonium picrate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
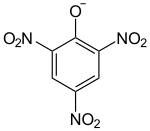
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ammonium picrate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 6 N 4 O 7 | |||||||||||||||
| Brief description |
explosive solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 246.14 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.72 g cm −3 |
|||||||||||||||
| Melting point |
Decomposition: 265 ° C |
|||||||||||||||
| solubility |
little in water (11 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Ammonium picrate is the ammonium salt of picric acid . Among other things, it was used as a military explosive for explosive charges during World War II . A common name is "Explosive-D".
Manufacturing
Ammonium picrate is obtained by saturating an aqueous solution of picric acid with ammonia . The product is red; when stored in the presence of water vapor , ammonium picrate changes into the more stable yellow form.
properties
- Enthalpy of formation : 389 kJ / mol (1581 kJ / kg)
- Formation energy: −1495 kJ / kg
- Oxygen balance: −52.0%
- Nitrogen content : 22.77%
- Normal gas volume : 999 l / kg
- Heat of explosion : 2954 kJ / kg
- Detonation speed : 7150 m / s
- Deflagration point : 320 ° C
- Lead block expansion : 280 cm 3 / 10g
- Impact sensitivity : no reaction up to 20 Nm
Individual evidence
- ↑ a b c d e Entry on ammonium picrate in the GESTIS substance database of the IFA , accessed on February 16, 2017(JavaScript required) .
- ↑ Not explicitly listed in Regulation (EC) No. 1272/2008 (CLP) , but with the specified labeling it falls under the group entry salts of picric acid in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ a b c d e f g h i J. Köhler, R. Meyer, A. Homburg: Explosivstoffe. 10., completely revised. Edition. Wiley-VCH, Weinheim 2008, ISBN 978-3-527-32009-7 .
literature
- J. Köhler, R. Meyer, A. Homburg: Explosivstoffe. 10., completely revised. Edition. Wiley-VCH, Weinheim 2008, ISBN 978-3-527-32009-7 , p. 20.

