Austenite (phase)
Austenite is the metallographic name for the face-centered cubic modification ( phase ) of pure iron and its mixed crystals .
Structure and properties
The austenitic phase (defined by the face-centered cubic lattice structure) occurs between temperatures of 1392 ° C and 911 ° C as γ-iron in pure iron. When it cools, it is formed from the δ-ferrite through a polymorphic transformation .
If carbon is added as an alloying element , the austenite is present as an embedded solid solution .
The face-centered cubic austenite lattice has octahedral gaps with a radius of 0.41 R. Despite the greater packing density, austenite is therefore able to dissolve significantly more carbon atoms than the krz ferrite lattice.
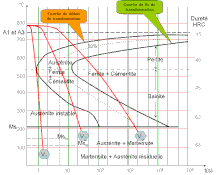
The maximum carbon solubility is 1147 ° C with 2.06% carbon, see iron-carbon diagram . The solubility of austenite decreases with temperature. At a temperature of 723 ° C, it is 0.8%.
The diffusion speed in austenite is lower than in ferrite.
The austenitic phase has paramagnetic properties (not magnetizable).
Stability and influences of alloying elements
When Fe-C alloys cool down, cementite precipitates along the ES line in the EKD ; this occurs for slow cooling speeds and results from the decreasing solubility of carbon in austenite.
When quenching austenite to room temperature with low carbon contents, the structure can be completely transformed into martensite . A diffusion-free flip-over process takes place, in which 2-car unit cells form a tetragonal-distorted unit cell. The carbon does not have time to diffuse, remains forcibly dissolved and thereby changes the lattice structure. With higher carbon contents and certain alloying elements (see below), a proportion of austenite, which increases with the carbon content, remains as retained austenite alongside martensite. Martensite start and martensite finish temperatures have decreased. With slow cooling from the austenite area, below 723 ° C, perlite , a lamellar structured mixture of ferrite and cementite, precipitates . The use of time-temperature conversion diagrams (TTT diagrams) makes it possible to determine which phase occurs at the end of a heat treatment for a specific alloy composition, depending on the cooling temperature and cooling rate.
The formation of the austenitic phase can be influenced by austenite or ferrite stabilizing elements.
Austenite-stabilizing alloying elements are nickel , carbon , cobalt , manganese and nitrogen (serves as a donkey bridge: NiCCoMnN turns on gamma). They stabilize or expand the area of existence of the γ single-phase space to higher and / or lower temperatures. Using the Schaeffler diagram , the nickel and chromium equivalents can be used to calculate which phase occurs when the cooling is sufficiently rapid. If the content of these alloying elements is sufficiently high, the entire steel structure remains metastable in the austenite state at room temperature if the martensite start temperature is below room temperature (austenitic steels). See also: Stainless steel # Austenitic steel grades
Ferrite-stabilizing alloy elements include chromium , aluminum , titanium , silicon , vanadium and molybdenum . They cause a closed γ-field. If there is a sufficient content of these alloying elements, the γ field is so severely constricted that the steel does not transform into austenite when heated (ferritic steel). The solubility of carbon in austenite is reduced by alloying elements, which shifts the eutectoid concentration .
See also
Individual evidence
- ↑ a b Wolfgang Bergmann: Werkstofftechnik 1. 7., revised edition, Carl Hanser Verlag, Munich 2013, ISBN 978-3-446-43581-0 , p. 215.
- ^ Wilhelm Domke: Materials science and material testing. 10th, improved edition, Cornelsen-Velhagen & Klasing, 2001, ISBN 3-590-81220-6 , p. 78.
- ↑ Bargel / Schulze (ed.): Material science. 11th edition, Springer, Berlin / Heidelberg 2012, ISBN 978-3-642-17716-3 , p. 176.
- ^ Editor of the Edelstahl-Vereinigung e. V. with Association of German Ironworkers (VDEh) (Ed.): Stainless steels. 2nd, revised edition, Verlag Stahleisen mbH, Düsseldorf 1989, ISBN 3-514-00333-5 , p. 21.
- ^ W. Schatt, H. Worch, Material Science 9th Edition, Willey-VCH Verlag GmbH & Co. KGaA, Weinheim 2003, ISBN 3527323236 , p. 172.
- ^ H. Oettel, H. Schuhmann, Metallography. 14th edition, Willey-VCH Verlag GmbH & Co. KGaA, Weinheim 2005, ISBN 3-527-30679-X , p. 401.
- ↑ a b Wilhelm Domke: Materials science and materials testing. 10th improved edition, Cornelsen-Velhagen & Klasing, 2001, ISBN 3-590-81220-6 , p. 160.
- ↑ Wolfgang Bergmann: Werkstofftechnik, 1st 7th revised edition, Carl Hanser Verlag, Munich 2013, ISBN 978-3-446-43581-0 , p. 227.
- ^ H. Oettel and H. Schuhmann, Metallography , 14th edition, Willey-VCH Verlag GmbH & Co. KGaA, Weinheim, 2005, p. 667, ISBN 3-527-30679-X