Balanol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
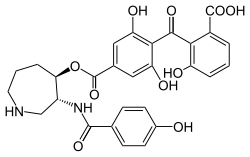
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Balanol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 28 H 26 N 2 O 10 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 551.17 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
decomposes above 180 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Balanol is a Azepan - derivative from the Hypocreomycetiden Verticillium balanoides , which in 1993 by Kulanthaivel was isolated. The compound is a potent inhibitor of protein kinase C and is regioisomeric to ophiocordin . Because of its pharmaceutical utility, balanol became a target of combinatorial chemistry .
Extraction and presentation
Balanol can be obtained from Boc-GABA-OH . In 2010 a new synthesis from 1,5-pentanediol was presented. This reacts in a Shi epoxidation and a nitrogen substitution to form a correctly configured balanol precursor.
Individual evidence
- ↑ Entry on Balanol. In: Römpp Online . Georg Thieme Verlag, accessed on March 29, 2014.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Entry in the Fungorum Index
- ↑ a b Phannarath Phansavath, Sébastien Duprat de Paule, Virginie Ratovelomanana-Vidal, Jean-Pierre Genêt: An Efficient Formal Synthesis of (-) - balanol by Using Catalyzed Asymmetric Hydrogenation ruthenium . In: European Journal of Organic Chemistry . tape 2000 , no. 23 December 2000, pp. 3903-3907 , doi : 10.1002 / 1099-0690 (200012) 2000: 23% 3C3903 :: aid-ejoc3903% 3E3.0.co; 2-q ( PDF ).
- ↑ Srinivasarao Yaragorla, Ramaiah Muthyala: Formal total synthesis of (-) - balanol: a potent PKC inhibitor . In: Tetrahedron Letters . tape 51 , no. 3 , January 2010, p. 467-470 , doi : 10.1016 / j.tetlet.2009.10.120 ( PDF ).
