Barium chlorite
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
| General | |||||||
| Surname | Barium chlorite | ||||||
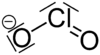
| Molecular formula | Ba (ClO 2 ) 2 | ||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 272.23 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| Melting point |
190 ° C (decomposition) |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Barium chlorite is a chemical compound from the group of chlorites .
Extraction and presentation
It is obtained in the laboratory by reacting chlorine dioxide with barium hydroxide and hydrogen peroxide :
properties
Barium chlorite is a solid which decomposes explosively when heated to 190 ° C. It also occurs as hydrate Ba (ClO 2 ) 2 · 3.5 H 2 O, which has a monoclinic crystal structure with the space group C 2 / c (space group no. 15) .
use
Barium chlorite can be used to make chlorous acid or sodium chlorite .
Individual evidence
- ↑ PG Urben: Bretherick's handbook of reactive chemical hazards . 2006, ISBN 0-12-372563-1 ( page 96 in the Google book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ G. Brauer (Ed.): Handbook of Preparative Inorganic Chemistry 2nd ed., Vol. 1, Academic Press 1963, p. 312.
- ↑ Barium chlorite hydrate, Ba (ClO2) 2 · 3.5H2O Acta Cryst. (2005). C61, i49-i50 doi: 10.1107 / S010827010500925X
- ↑ M. Mazumdar: Rudiments of Chemistry . 1st edition. 2006, ISBN 81-89781-53-7 ( page 382 in Google book search).
