Barium germanide
| Crystal structure | |||||||
|---|---|---|---|---|---|---|---|
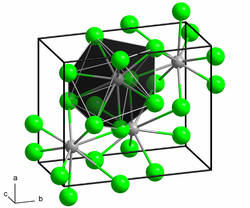
|
|||||||
| __ Ge 4− __ Ba 2+ | |||||||
| Crystal system | |||||||
| Space group |
Pnma (No. 62) |
||||||
| Lattice parameters |
|
||||||
| General | |||||||
| Surname | Barium germanide | ||||||
| other names |
|
||||||
| Ratio formula | Ba 2 Ge | ||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 347.29 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| Melting point |
1000 ° C |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Barium germanide , Ba 2 Ge, is an inorganic compound of barium with germanium .
Extraction
Barium germanide was produced by melting the two elements together under an argon atmosphere at 1200 ° C.
properties
The intermetallic Zintl phase crystallizes in the anti- PbCl 2 type in an orthorhombic crystal system with the space group Pnma (space group no. 62) . The lattice parameters are a = 8.38 Å , b = 5.48 Å and c = 10.04 Å.
Individual evidence
- ^ Richard C. Ropp: Encyclopedia of the Alkaline Earth Compounds . Newnes, 2012, ISBN 0-444-59553-8 , pp. 423 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b K. Turban, Herbert Schäfer: Notes: Representation and crystal structure of Ba 2 Ge / Preparation and Crystal structure of Ba 2 Ge . In: Journal of Nature Research B . April 1973, p. 220-222 , doi : 10.1515 / znb-1973-3-427 .