Biosurfactants

Biosurfactants are surfactant molecules of microbial origin that can be produced on the basis of vegetable oil and sugar substrates. Just like surfactants of non-microbial origin, biosurfactants are amphiphilic , i.e. they have both a hydrophobic and a hydrophilic part of the molecule. Thanks to their amphiphilic character, they are used to reduce surface tension or interfacial tension.
Biosurfactants are currently only produced and used in very small quantities, as they are not economically competitive compared to synthetic surfactants based on petroleum. However, their critical micelle concentration (CMC) is technically comparable with conventional nonionic surfactants.
properties
Surface-active substances of microbial origin can be divided into chemically different groups. The glycolipids , lipopeptides and lipoamino acids , lipoproteins and lipopolysaccharides as well as phospholipids , mono- and diglycerides and fatty acids are of particular importance.
The glycolipids are the most common group of low molecular weight biosurfactants and can be divided into:
- Rhamnose lipids
- Sophorose lipids
- Trehalose and other mycolic acid containing glycolipids
- Cellobiose and mannosylerythritol lipids
The hydrophilic “molecular head” can be both non-ionic and ionic in nature. These include mono-, di- and polysaccharides as well as carboxylic acid, amino acid and peptide groups. The hydrophobic “molecular tail” usually consists of unsaturated, saturated or hydroxylated fatty acids.
Biotechnological production of biosurfactants
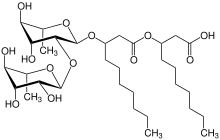
Biosurfactants produced with the help of bacteria or fungi are of great interest to industrial biotechnology . One advantage over the petrochemical production of surfactants is that biosurfactants can be produced on the basis of renewable raw materials (e.g. vegetable oils , sugar in the form of syrup or molasses ). A high degree of surfactant efficiency and good biodegradability are further reasons that speak for the use of biosurfactants.
The biotechnological production of biosurfactants has been established for some time, but due to the high production costs they have only been used in niche areas so far. In doing so, ex. Rhamnose lipids (rhamnolipid) mainly produced by Pseudomonas aeruginosa , surfactin from Bacillus subtilis , emulsan from Acinetobacter calcoaceticus and liposan from Candida lipolytica as well as sophorose lipid (sophorolipid) from Torulopsis bombicola . Cellobiose lipids can be formed in the presence of alkanes or triglycerides by the corn smut ( Ustilago maydis ) while corynomycolates and trehalose lipids are produced by bacteria of the genera Corynebacterium and Arthrobacter . Particularly high yields are achieved in the production of sophorolipids with more than 400 g / l suspension, while with most other biosurfactants up to 110 mg / l suspension can be achieved.
The process optimization is a focus of current biotechnological research on biosurfactants. Due to the very good surfactant properties, there is also the problem of foaming in particular . There are good chemical anti-foam agents (especially silicone oils ), but these can have an impact on the product quality. In current research, alternatives are therefore being tested, such as mechanical foam destruction.
Some biosurfactant-producing organisms such as Pseudomonas aeruginosa , the sole producer of significant amounts of rhamnolipid, are opportunistic pathogens and are therefore classified as potentially dangerous microorganisms. Dealing with them is linked to appropriate technical measures and is very complex, alternative producers are being researched. The surfactin from Bacillus subtilis has very good surfactant properties, but due to its haemolytic effect it is not used.
Application potential of biosurfactants
The use of biosurfactants basically spans the areas of application of chemically synthesized surfactants . Due to the potentially better biodegradability of the biosurfactants, special, environmentally relevant areas of application can be opened up that are not possible for chemically synthesized surfactants. Corresponding areas of application for biosurfactants are e.g. Uses in the field of bioremediation or environmental remediation (e.g. treatment with trehalose dicorynomycolate after major oil disasters ) and tertiary oil production (" Enhanced Oil Recovery "). The main applications, however, correspond to those of synthetic surfactants in household and cleaning products, cosmetics , medicine , in food process engineering and in agriculture and crop protection, in which surfactants from oleochemical production (direct chemical conversion of vegetable oils) are also used.
Rhamnolipids in particular are used in household cleaners (Henkel, Ecover ), while sophorolipids are mainly used in skin creams in Japan.
Individual evidence
- ↑ a b c R. Hausmann, B. Hörmann, MM Müller, V. Walter, C. Syldatk: Production of microbial rhamnolipids. Abstract for the talk at the ProcessNet annual conference / 27. Annual meeting of biotechnologists, published in CIT - Chemie Ingenieur Technik. Volume 81, No. 8, 2009, p. 1212.
- ↑ a b c d Rolf D. Schmid: Pocket Atlas of Biotechnology and Genetic Engineering. 2nd Edition. Wiley-VCH, Weinheim 2006, ISBN 3-527-31310-9 , pp. 58-59.
- ↑ F. Leitermann: Biotechnological production of microbial rhamnolipids. University of Karlsruhe (TH), Karlsruhe 2008.
literature
- S. Lang, W. Trowitzsch-Kienast: Biosurfactants. BG Teubner, Stuttgart / Leipzig / Wiesbaden 2002, ISBN 3-519-03615-0 .
- G. Georgiou, SC Lin et al: Surface-Active Compounds from Microorganisms. In: Bio-Technology. Volume 10, No. 1, 1992, pp. 60-65.
- F. Leitermann: Biotechnological production of microbial rhamnolipids. University of Karlsruhe (TH), Karlsruhe 2008, ISBN 978-3-86644-277-1 .
- S.-C. Lin: Bisurfactants: Recent Advances. In: J. Chem. Tech. Biotechnol. 66, 1996, pp. 109-120.
Web links
- Karlsruhe Institute of Technology: Process development for the production of biosurfactants (project) ( Memento from June 15, 2010 in the Internet Archive )
- Andrea Hoferichter: Little helpers for environmentally friendly detergents. In: Berliner Zeitung . April 26, 2007, accessed June 14, 2015 .
