Brusselsator
The Brusselsator is a simple model for describing chemical oscillators . The Brusselsator was developed by Ilya Prigogine and René Lefever at the Université Libre de Bruxelles in Belgium, hence the name.
description
Four hypothetical reaction equations form a simple model that reflects all the phenomena of chemical oscillators (such as the Belousov-Zhabotinsky reaction ). A similar model system was derived in 1985 at the Humboldt University in Berlin by simplifying a real existing reaction system.
| I. | A. | → | X | |
| II | B + X | → | Y + C | |
| III | 2X + Y | → | 3X | ( autocatalytic ) |
| IV | X | → | D. | |
|
|
||||
| Σ (I-IV once each) | A + B | → | C + D | |
The reaction rates are reflected by the constants k 1 to k 4 , the concentrations of A and B are kept constant, and the products C and D are continuously removed.
The concentrations of X and Y are sensitive to small perturbations and quickly reach an oscillating state if the overall reaction is far from equilibrium . So you have a thermodynamically open system and you can set up two rate equations for the concentration of X and Y:
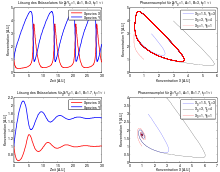
These differential equations can be solved numerically . The figure opposite shows some solutions. Depending on the choice of the free parameters k 1 A , k 2 B , k 3 and k 4 , different behavior results: in the upper case one sees stable oscillations, while in the lower case with a different choice of parameters the concentrations X (t) and Y ( t) strive for a fixed point in phase space .
Analysis of stability
As shown above, depending on the parameterization, the Brusselsator has stable oscillations as a solution or strives towards a fixed point in phase space.
This fixed point results from
to:
With the help of the linear stability analysis it can also be shown that this fixed point becomes unstable if:
In this case the trajectories tend towards a limit cycle in phase space and the system carries out the oscillations shown.
As a reaction-diffusion model

The model can also be extended to a reaction-diffusion model and, if the correct parameters are selected, chemical waves are obtained as a solution, as shown on the right.
The differential equations are a diffusion share or expanded and become:
Is in here
- a point in space
- the diffusion coefficient
- the Laplace operator , i.e. the sum of the second spatial derivatives in Cartesian coordinates .
literature
- Dilip Kondepudi, Ilya Prigogine: Modern Thermodynamics. From Heat Engines to Dissipative Structures. John Wiley & Sons, Weinheim, New York 1998.
Individual evidence
- ↑ RJ Field: An oscillating response. In: Chemistry in our time, 7th year 1973, No. 6, pp. 171–176, doi: 10.1002 / ciuz.19730070603 .
- ↑ K. Bar-Eli: The minimal bromate oscillator simplified , J. Phys. Chem., 1985, doi: 10.1021 / j100259a030 .
- ↑ a b c Dilip Kondepudi, Prigogine : Modern Thermodynamics. From Heat Engines to Dissipative Structures. John Wiley & Sons, Chichester et al. 1998, ISBN 0-471-97393-9 .
Web links
- Jan Krieger: Reaction Diffusion Systems (PDF; 2.5 MB), 2004.










