Belousov-Zhabotinsky reaction

The Belousov-Zhabotinsky reaction ( BZR or BZ reaction ) is the classic example of a homogeneous chemical oscillator . It is often used to illustrate emergent and chaotic systems . It is a system of several chemical reactions that shows a temporal oscillation or a self-organized spatial structure, depending on the experimental conditions, which is actually unusual for chemical reactions. Initially, the reaction was mistaken for a measurement error or artifact , as the second law of thermodynamics seemed to forbid such a process. This theorem of physics says that a disordered state cannot develop on its own (i.e. without the supply of external energy) a more ordered state. However, this theorem is not applicable here, since it only applies to closed systems in thermal equilibrium. The Belousov-Zhabotinsky reaction, however, is a dissipative reaction that takes place far from thermal equilibrium and therefore shows this extraordinary behavior.
Classically, the Belousov-Zhabotinsky reaction is carried out in a Petri dish (see figure on the right) because the pattern can be clearly seen with an overhead projector, for example. It spreads like circular waves .
The principle of the chemical oscillator can also be demonstrated with other reaction systems, such as the so-called Ioduhr ( Briggs-Rauscher reaction ).
history
Around 1950 Boris Pavlovich Belousov (Борис Павлович Белоусов) discovered the Belousov-Zhabotinsky reaction by chance. During the oxidation of citric acid with bromate sulfuric acid solution and cerium ions as a catalyst, he was able to observe a periodic change in the color of the solution between yellow and colorless. Since this observation seemed too improbable for the same reason as with Bray, Belousov only managed to publish a short article about it in 1959. HE Schnoll recognized the importance of this reaction and commissioned Anatoly Markowitsch Schabotinsky (Анатолий Маркович Жаботинский) to investigate the phenomenon described, which he published in 1964.
Slowly, non-Russian scientists became interested in oscillating reactions, and extensive research into the phenomena related to them began. For example, spatial structures (circular patterns) were discovered that can form in a thin layer of a solution of the Belousov-Zhabotinsky reaction.
In 1977 Ilya Prigogine received the Nobel Prize in Chemistry for his important research in the field of thermodynamics . He investigated systems that were far from equilibrium ( dissipative structures ), both in chemistry (the Belousov-Zhabotinsky reaction belongs to this class of processes) and in physics, in biology (such as the Lotka-Volterra model for predator prey Systems ) and sociology. After this Nobel Prize, Belousov ( posthumously ), Schabotinsky and with them Zaikin, Krinsky and Ivanizki were honored with the Lenin Prize , the highest scientific award in the Soviet Union, in 1980 .
Reactions
Solutions of four substances, potassium bromate , malonic acid , potassium bromide and concentrated sulfuric acid , as well as ferroin or another redox indicator are involved in the reaction . During the reaction, the state of the indicator changes constantly between the reduced and the oxidized form, which causes a typical color change. With ferroin as an indicator, the color changes between blue ( ferriin , with Fe 3+ ) and red (ferroin, with Fe 2+ ), with cerium between yellow (Ce 4+ ) and colorless (Ce 3+ ), with manganese between red ( Mn 3+ ) and colorless (Mn 2+ ). The reaction does not take indefinitely, since both malonic acid and bromate are consumed.
During the reaction, three different processes (A, B, and C) occur, each with several reactions. Process A is non-radical, the redox indicator is not involved. Essentially, bromide is consumed and converted to monobromomalonic acid. During this reaction, bromous acid is formed , which is further converted.
- Overall reaction of process A
If a lot of bromide is consumed, this enables the reactions of process B to take place. This is radical and takes place with the redox indicator. In a first reaction, bromous acid acts as an autocatalyst (see autocatalysis ), whereby the concentration of bromous acid doubles per reaction.
At higher concentrations of bromous acid, this reacts to hypobromous acid , so that an overall reaction for process B of
results.
In order for an oscillation to be possible, there has to be another reaction in which the bromide used is regenerated. This is process C, in which malonic acid (H 2 Mal), monobromomalonic acid (HBrMal), hypobromite and the redox indicator react with each other to form tartronic acid [hydroxymalonic acid, HOCH (COOH) 2 ] with bromide formation .
More bromide is produced by the decomposition of tartronic acid with bromate to carbon dioxide and water.
Model for the reaction process
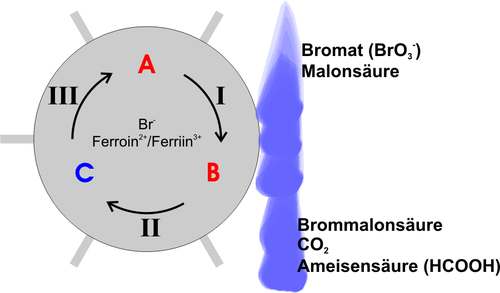
A simple model for the BZR will be described below. The following picture serves as an illustration:
In the initial state A , in addition to the starting materials (bromate, malonic acid), the solution mainly contains bromide and ferroin. Reaction I can now take place, consuming the bromide and brominating malonic acid. The system changes to state B , in which there is almost no bromide. Even in the smallest concentrations, the bromide inhibits the course of reaction II , so that it does not play a role at the beginning. But now it can run off and oxidize the ferroin, which leads to a color change in the solution to blue. The system is now in state C , in which the solution contains ferriin and no bromide. Reaction III can now take place and reduces the ferriin again to ferroin, whereby bromide is also formed back again. In addition, still produced formic acid as a product. This state corresponds to the initial state A again .
This model is very simplified. There are works in which up to 20 partial equations are used in order to achieve a very precise modeling of the system.
Mathematical models
Various mathematical models have been developed to depict the behavior of chemical oscillators. These include:
The Brusselsator is very simple, but physically unrealistic. However, it provides results that are very close to the BZR (see figure). In addition, the system is relatively simple and can be analyzed mathematically well. Often, models for the BZR can be simulated with a cellular machine and so models for the spatial patterns in the BZR are achieved.
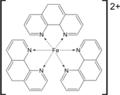
Structural formulas of the substances involved
literature
- BP Belousov: A Periodic Response and its Mechanism. In: L. Kuhnert, U. Niedersen (Ed.): Self-organization of chemical structures. Harri Klein Verlag, Frankfurt am Main 1981, pp. 73-82.
- AM Zhabotinsky: A periodic oxidation reaction in the liquid phase. In: L. Kuhnert, U. Niedersen (Ed.): Self-organization of chemical structures. Harri Klein publishing house, Frankfurt am Main 1964, pp. 83-89.
- Irving R. Epstein, Kenneth Kustin, Patrick de Kepper, Miklós Orban: Oscillating chemical reactions. In: Spectrum of Science. , No. 5, 1983, pp. 98-107.
- Richard J. Field: The Experiment: An Oscillating Response. In: Chemistry in Our Time. 7, No. 6, 1973, pp. 171-176, doi : 10.1002 / ciuz.19730070603 .
- Richard J. Field, Friedmann W. Schneider: Oscillating chemical reactions and nonlinear dynamics. In: Chemistry in Our Time. 22, No. 1, 1988, pp. 17-29, doi : 10.1002 / ciuz.19880220104 .
- Ulrich F. Franck: Chemical Oscillations. In: Angewandte Chemie . 90, No. 1, 1978, pp. 1-16, doi : 10.1002 / ange.19780900104 .
- Jearl Walker: Oscillating Chemical Reactions. In: Spectrum of Science . 5, No. 231, 1980, pp. 131-137.
Web links
- Jan Krieger: Oscillating chemical reactions using the example of the Belousov-Zhabotinsky reaction . May 5, 2001 (technical work in the national youth research competition 2001).
- Video: The Zhabotinsky reaction as a model of pattern formation . Institute for Scientific Film (IWF) 1983, made available by the Technical Information Library (TIB), doi : 10.3203 / IWF / C-1473 .
Individual evidence
- ↑ Ilya Prigogine: From Being to Becoming - Time and Complexity in the Sciences , Piper 1992.
- ^ Richard J. Field, Friedmann W. Schneider: Oscillating chemical reactions and nonlinear dynamics. In: Chemistry in Our Time. 22, No. 1, 1988, pp. 17-29, doi : 10.1002 / ciuz.19880220104 .
- ↑ Richard J. Field, Richard M. Noyes: Oscillations in chemical systems. IV. Limit cycle behavior in a model of a real chemical reaction. In: The Journal of Chemical Physics. 60, No. 5, 1974, pp. 1877-1884, doi : 10.1063 / 1.1681288 .