Caspases
| Caspases | ||
|---|---|---|
| Enzyme classification | ||
| EC, category | 3.4.22. , Cysteine protease | |
| MEROPS | C14 | |
| Response type | Asp - / - | |
Caspases ( English c ysteinyl- a spartate s pecific p red ase ) are a group of cysteine proteases that cut target proteins at a peptide bond C -terminal of aspartate , which is where the name comes from. In animals, caspases are the most important enzymes involved in apoptosis , the programmed cell death.
properties
Caspases are essential for the correct development of a living being, but also for the response of a cell to severe damage (e.g. by radiation) or to an infection by intracellular pathogens such as e.g. B. Viruses .
The caspases are part of an enzyme cascade to initiate apoptosis. To trigger cell death, initiator caspases (e.g. caspase-8 and 9) are first activated. These in turn split the pro form ( precursor form ) of downstream caspases ( effector caspases, including caspase 3, 7, 6), which split the cell's own proteins such as actin and lamin . Another important function of the effector caspases is the activation of a nuclease that cleaves the nuclear DNA between the histones during apoptosis , which reveals a DNA ladder in agarose gel electrophoresis .
In addition to apoptosis , caspases are involved in the development of erythrocytes and myoblasts , and in synaptic plasticity .
Defects in caspases are involved in the development of tumors . Some viruses and intracellular bacteria try to prevent caspase activation in the course of an immune evasion. Some viruses use caspases to activate their own proteins. Other diseases with disorders of the caspase cascade are z. As protein misfolding diseases such as Alzheimer's disease , Parkinson's disease , Huntington's disease and amyotrophic lateral sclerosis , and stroke , ischemia , heart failure , systemic lupus erythematosus , autoimmune lymphoproliferative syndrome , rheumatoid arthritis and thyroiditis .
Types
So far, twelve different caspases have been described in humans. These are divided into three groups: proinflammatory caspases, initiator caspases and effector caspases. Initiator caspases (e.g. CASP2 , CASP8 , CASP9 and CASP10 ) cut effector caspases into their active form. Effector caspases (e.g. CASP3 , CASP6 , CASP7 ) cut other cellular proteins of apoptosis. The activation is inhibited by caspase inhibitors. Casp6 also plays a role in the derepression of the immune system in the early phase of infection and in the cleavage of huntingtin in Huntington's disease and amyloid precursor protein in Alzheimer's disease .
CASP1 , CASP4 and CASP5 are inflammatory caspases and are involved in the maturation of T cells . CASP4 and CASP5 are in some forms of vitiligo and related NALP1-associated autoimmune diseases overexpressed .
Caspase cascade
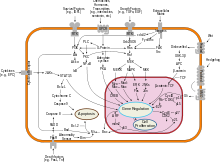
The activity of caspases is not change the gene expression selected, but by post-translational modification via proteolysis. Initiator caspases cut further caspases into their active form, which results in an exponential signal amplification. As a result of the proteolytic activation of the caspases, more and more proteins are broken down. This enables apoptosis to be initiated more quickly. With initiator caspases, the prodomains to be separated are longer than with effector caspases. The pro-domain of the initiator caspases contains a CARD domain (e.g. Caspase-2 and -9) or a death effector domain (DED) (for Caspase-8 and -10). When activated, the initiator caspases group together and additionally activate each other through mutual proteolysis.
The initiator caspases can be activated by various enzymes:
- Granzyme B is used by cytotoxic T cells and NK cells distributed , thereby caspase-3 and -7 is activated.
- death receptors such as Fas receptors , TRAIL receptors and TNF receptors , which activate caspase-8 and -10.
- The apoptosome (regulated by cytochrome c and the Bcl-2 family ) activates caspase-9.
The effector caspases cut various cellular proteins:
- Lamine
- ICAD / DFF45 ( inhibitor of caspase activated DNase or DNA fragmentation factor 45 )
- PARP (poly-ADP-ribose polymerase)
- PAK2 ( P 21-activated kinase 2 )
The protein ICAD / DFF45 inhibits caspase-activated DNase (CAD). Proteolysis by effector caspases inactivates this inhibition, whereby the DNA in the cell is fragmented. Caspase-1 and -3 in macrophages are inhibited by the DNA-binding proteins HIN-200 (synonym p202) and activated by AIM2 (synonym p210).
history
H. Robert Horvitz discovered the involvement of the gene ced-3 (from Caenorhabditis elegans death gene ) in programmed cell death. Horvitz and his colleague Junying Yuan discovered in 1993 the similarity of ced-3 to the mammalian cysteine protease interleukin-1-beta converting enzyme (ICE, now known as caspase-1). The nomenclature of caspases was adopted in 1996 because, due to the simultaneous discovery in several research groups, different names were often used, e.g. B. Caspase 3 was described as CPP32 , Apopain and Yama . The caspases have been renamed in the order in which they were discovered.
Web links
- Jennifer McDowall / Interpro: Protein Of The Month: Caspases. (engl.)
- MeSH caspases
Individual evidence
- ↑ M. Kurokawa, S. Kornbluth: Caspases and kinases in a death grip. In: Cell. Volume 138, Number 5, September 2009, pp. 838-854, doi: 10.1016 / j.cell.2009.08.021 . PMID 19737514 . PMC 3390419 (free full text).
- ↑ M. Lamkanfi, et al. : Caspases in cell survival, proliferation and differentiation . In: Cell Death and Differentiation . 14, No. 1, January 2007, pp. 44-55. doi : 10.1038 / sj.cdd.4402047 . PMID 17053807 . Retrieved February 28, 2011.
- ^ Z. Li, M. Sheng: Caspases in synaptic plasticity. In: Molecular brain. Volume 5, 2012, p. 15, doi: 10.1186 / 1756-6606-5-15 . PMID 22583788 . PMC 3366905 (free full text).
- ↑ MV Fiandalo, N. Kyprianou caspase control: Protagonists of cancer cell apoptosis. In: Experimental oncology. Volume 34, Number 3, October 2012, pp. 165-175, PMID 23070001 . PMC 3721730 (free full text).
- ↑ M. Olsson, B. Zhivotovsky: Caspases and cancer. In: Cell death and differentiation. Volume 18, number 9, September 2011, pp. 1441-1449, doi: 10.1038 / cdd.2011.30 . PMID 21455218 . PMC 3178435 (free full text).
- ↑ E. Frejlich, J. Rudno-Rudzińska, K. Janiszewski, L. Salomon, K. Kotulski, O. Pelzer, Z. Grzebieniak, R. Tarnawa, W. Kielan: Caspases and their role in gastric cancer. In: Advances in clinical and experimental medicine: official organ Wroclaw Medical University. Volume 22, Number 4, 2013 Jul-Aug, pp. 593-602, PMID 23986221 .
- ↑ AM Fuentes-González, A. Contreras-Paredes, J. Manzo-Merino, M. Lizano: The modulation of apoptosis by oncogenic viruses. In: Virology journal. Volume 10, 2013, p. 182, doi: 10.1186 / 1743-422X-10-182 . PMID 23741982 . PMC 3691765 (free full text).
- ^ NH Philip, IE Brodsky: Cell death programs in Yersinia immunity and pathogenesis. In: Frontiers in cellular and infection microbiology. Volume 2, 2012, p. 149, doi: 10.3389 / fcimb.2012.00149 . PMID 23226685 . PMC 3510641 (free full text).
- ^ A. Richard, D. Tulasne: Caspase cleavage of viral proteins, another way for viruses to make the best of apoptosis. In: Cell death & disease. Volume 3, 2012, p. E277, doi: 10.1038 / cddis.2012.18 . PMID 22402601 . PMC 3317351 (free full text).
- ^ A b c Rona K. Graham, Dagmar E. Ehrnhoefer, Michael R. Hayden: Caspase-6 and neurodegeneration . In: Trends in Neurosciences . tape 34 , no. 12 , December 1, 2011, p. 646-656 , doi : 10.1016 / j.tins.2011.09.001 , PMID 22018804 ( cell.com [accessed July 12, 2017]).
- ↑ B. Favaloro, N. Allocati, V. Graziano, C. Di Ilio, V. De Laurenzi: Role of apoptosis in disease. In: Aging. Volume 4, Number 5, May 2012, pp. 330-349, PMID 22683550 . PMC 3384434 (free full text).
- ↑ HUGO Gene Nomenclature Committee ( Memento of the original from September 8, 2013 in the Internet Archive ) Info: The archive link was automatically inserted and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ LL Fava, FJ Bock, S. Geley, A. Villunger: Caspase-2 at a glance. In: Journal of cell science. Volume 125, Pt 24 December 2012, pp. 5911-5915, doi: 10.1242 / jcs.115105 . PMID 23447670 .
- ↑ Hiroshi Kobayashi, Anna Nolan, Bushra Naveed, Yoshihiko Hoshino, Leopoldo N. Segal: Neutrophils Activate Alveolar Macrophages by Producing Caspase-6 - Mediated Cleavage of IL-1 Receptor-Associated Kinase-M . In: The Journal of Immunology . tape 186 , no. 1 , January 1, 2011, p. 403-410 , doi : 10.4049 / jimmunol.1001906 , PMID 21098228 ( jimmunol.org [accessed July 12, 2017]).
- ↑ Alexander Bartel, André Göhler, Verena Hopf, Katrin Breitbach: Caspase-6 mediates resistance against Burkholderia pseudomallei infection and influences the expression of detrimental cytokines . In: PLOS ONE . tape 12 , no. 7 , July 7, 2017, p. e0180203 , doi : 10.1371 / journal.pone.0180203 ( plos.org [accessed July 12, 2017]).
- ^ PK Gregersen: Modern genetics, ancient defenses, and potential therapies . In: N Engl J Med. . 356, No. 12, March 22, 2007, pp. 1263-6. doi : 10.1056 / NEJMe078017 . PMID 17377166 . [ PMID 17377166 ]
- ↑ P. Li, et al. : Mitochondrial Activation of Apoptosis . In: Cell . 116, No. 2 Suppl, January 2004, pp. 57-59. doi : 10.1016 / S0092-8674 (04) 00031-5 . PMID 15055583 . Retrieved November 6, 2006.
- ↑ TL Roberts, A. Idris, JA Dunn, GM Kelly, CM Burnton, S. Hodgson, LL Hardy, V. Garceau, MJ Sweet, IL Ross, DA Hume, KJ Stacey: HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. In: Science. Volume 323, number 5917, February 2009, pp. 1057-1060, doi: 10.1126 / science.1169841 . PMID 19131592 .
- ^ NN Danial, Korsmeyer, SJ: Cell Death: Critical Control Points . In: Cell . 116, No. 2, January 2004, pp. 205-219. doi : 10.1016 / S0092-8674 (04) 00046-7 . PMID 14744432 . Retrieved November 6, 2006.
- ↑ Yuan, J et al .: The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme . In: Cell . 75, No. 4, 1993, pp. 641-652. doi : 10.1016 / 0092-8674 (93) 90485-9 . PMID 8242740 .
- ↑ J. Yuan, Horvitz, HR: A First Insight into the Molecular Mechanisms of Apoptosis . In: Cell . 116, No. 2 Suppl, January 2004, pp. 53-56. doi : 10.1016 / S0092-8674 (04) 00028-5 . PMID 15055582 . Retrieved November 6, 2006.
- ↑ ES Alnemri, DJ Livingston, DW Nicholson, G. Salvesen, NA Thornberry, WW Wong, J. Yuan: Human ICE / CED-3 protease nomenclature. In: Cell. Volume 87, Number 2, October 1996, p. 171, PMID 8861900 .