Chloroformic acid
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
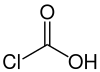
|
|||||||||||||
| General | |||||||||||||
| Surname | Chloroformic acid | ||||||||||||
| other names |
Chlorinated acid |
||||||||||||
| Molecular formula | CHClO 2 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 80.47 g mol −1 | ||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Chloroformic acid is a chemical compound ; it is the primary chloride of carbonic acid . The term 'chloroformic acid' is therefore, strictly speaking, incorrect and should be chlorocarbonic acid. The corresponding stable dichloride is known as phosgene .
The compound is only known in the form of its esters , which are formed when the secondary chloride of carbonic acid, carbon oxychloride, acts on alcohols . These are volatile, unpleasant-smelling liquids that break down with water into alcohol, carbonic acid and hydrochloric acid and form neutral carbonic acid esters with anhydrous alcohol and urethanes with ammonia . The class of substances discovered by Jean-Baptiste Dumas has achieved great preparative importance and has been extensively investigated. Chloroformic acid esters (e.g. benzyl chloroformate ) are of particular importance for peptide syntheses since they are used to introduce N-protective groups.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Eberhard Breitmaier, Günther Jung: Organic Chemistry, 7th complete revision. u. exp. Edition 2012 Basics, classes of compounds, reactions, concepts, molecular structure, natural substances, synthesis planning, sustainability . Georg Thieme Verlag, 2014, ISBN 3-13-159987-1 , p. 431 ( limited preview in Google Book search).
- ↑ Zeno.org: Chloroformic Acid - Zeno.org , accessed January 3, 2018
- ↑ Houben-Weyl Methods of Organic Chemistry Vol. E 4, 4th Edition Supplement Carbonic Acid Derivatives . Georg Thieme Verlag, 2014, ISBN 3-13-181144-7 , p. 16 ( limited preview in Google Book search).
- ↑ Hans Peter Latscha, Uli Kazmaier: Chemistry for Biologists . Springer-Verlag, 2016, ISBN 978-3-662-47784-7 , p. 588 ( limited preview in Google Book search).