Coumestrol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
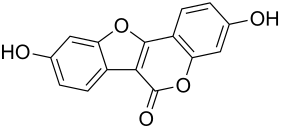
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Coumestrol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 15 H 8 O 5 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 268.22 g mol −1 | ||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Coumestrol is a chemical compound that belongs to the coumarin derivatives. It is a dihydroxy - derivative (chemistry) of Coumestans . Coumestans are estrogen- like substances ( phytoestrogens ) that are produced by some types of plants. Coumestrol binds to and activates the estrogen receptors hERα and β.
Occurrence
Coumestrol was first discovered in 1957 by EM Bickoff in alfalfa .
It was subsequently detected in many other plants such as legumes , soybeans , Brussels sprouts and spinach . Clover and soybeans contain the highest concentrations.
Individual evidence
- ↑ a b Coumestrol data sheet from Sigma-Aldrich , accessed on March 23, 2011 ( PDF ).
- ↑ Miksicek RJ: Interaction of naturally occurring nonsteroidal estrogens with expressed recombinant human estrogen receptor . In: J. Steroid Biochem. Mol. Biol . 49, No. 2-3, June 1994, pp. 153-60. PMID 8031711 .
- ↑ Morito K, Aomori T, Hirose T, et al : Interaction of phytoestrogens with estrogen receptors alpha and beta (II) . In: Biol Pharm Bull . 25, No. 1, January 2002, pp. 48-52. PMID 11824555 .
- ^ Cos P, De Bruyne T, Apers S, Vanden Berghe D, Pieters L, Vlietinck AJ: Phytoestrogens: recent developments . In: Planta Med . 69, No. 7, July 2003, pp. 589-99. doi : 10.1055 / s-2003-41122 . PMID 12898412 .
- ↑ EM Bickoff, AN Booth, RL Lyman, AL Livingston, CR Thompson, and F. Deeds: Coumestrol, a New Estrogen Isolated from Forage Crops . In: Science . 126, No. 3280, 1957, pp. 969-970. doi : 10.1126 / science.126.3280.969-a . PMID 13486041 .
- ↑ Amr Amin and Michael Buratovich, "The Anti-Cancer Charm of Flavonoids: A Cup-of-Tea Will Do!. In: Recent Patents on Anti-Cancer Drug Discovery . 2, No. 2, 2007, pp. 109-117 . doi : 10.2174 / 157489207780832414 . PMID 18221056 .

