Diethyl germanium dichloride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
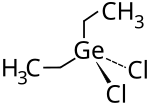
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Diethyl germanium dichloride | |||||||||||||||
| other names |
Dichlorodiethylgerman |
|||||||||||||||
| Molecular formula | (C 2 H 5 ) 2 GeCl 2 | |||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 201.67 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.372 g cm −3 (25 ° C) |
|||||||||||||||
| Melting point |
172.8 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.471 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Diethylgermanium dichloride is a chemical compound from the group of organic germanium compounds .
Extraction and presentation
Diethylgermanium dichloride can be obtained by reacting chloroethane with germanium at 625 K over copper.
It is also possible to display it by reacting tetraethylgermanium with germanium (IV) chloride .
properties
Diethylgermanium dichloride is a colorless liquid that decomposes on contact with water.
use
Diethylgermanium dichloride is used as an intermediate in the manufacture of other chemical compounds.
Individual evidence
- ↑ a b c d e f g data sheet Diethylgermanium dichloride, 97% from Sigma-Aldrich , accessed on October 19, 2015 ( PDF ).
- ^ EG Rochow, EW Abel: The Chemistry of Germanium: Tin and Lead . Elsevier, 2014, ISBN 978-1-4831-8758-7 , pp. 34 ( books.google.de ).
- ↑ a b c data sheet diethylgermanium dichloride from AlfaAesar, accessed on October 5, 2017 ( PDF )(JavaScript required) .
- ^ RK Agarwal, B. Tech: Kirshna's Engineering Chemistry: (UP) (Theory and Practicals) . Krishna Prakashan Media, ISBN 978-81-87224-92-1 , pp. 309 ( books.google.de ).
- ↑ Jane E. Macintyre: Dictionary of Organometallic Compounds . CRC Press, 1994, ISBN 978-0-412-43060-2 , pp. 1715 ( books.google.de ).
- ^ Wiley Online Library: Contributions to the chemistry of phosphorus. 123. Synthesis and properties of the diphosphagermiranes (t-BuP) 2GePh2 and (t-BuP) 2GeEt2 . doi : 10.1002 / zaac.19835030802 ( wiley.com ).

