Ethyl germanium trichloride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
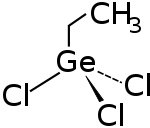
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ethyl germanium trichloride | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 2 H 5 Cl 3 Ge | |||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 208.06 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.60 g cm −3 |
|||||||||||||||
| Melting point |
<−33 ° C |
|||||||||||||||
| boiling point |
144 ° C |
|||||||||||||||
| Refractive index |
1.474 (25 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Ethylgermanium trichloride is a chemical compound from the group of organic germanium compounds .
Extraction and presentation
Ethyl germanium trichloride can be obtained by reacting germanium (IV) chloride with tetraethyl tin . The compound can also be represented by the reaction of ethyl chloride with germanium and copper .
properties
Ethyl germanium trichloride is a colorless liquid that reacts with water.
use
Ethyl germanium trichloride can be used as a polymerization catalyst.
Individual evidence
- ↑ AMERICAN ELEMENTS ®: Ethylgermanium Trichloride | AMERICAN ELEMENTS ® , accessed October 8, 2017.
- ^ A b Carl L. Yaws: The Yaws Handbook of Physical Properties for Hydrocarbons and Chemicals Physical Properties for More Than 54,000 Organic and Inorganic Chemical Compounds, Coverage for C1 to C100 Organics and Ac to Zr Inorganics . Gulf Professional Publishing, 2015, ISBN 978-0-12-801146-1 , pp. 17 ( limited preview in Google Book search).
- ↑ a b c d Jane E. Macintyre: Dictionary of Organometallic Compounds . CRC Press, 1994, ISBN 978-0-412-43060-2 , pp. 1710 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ A b Michael Dub: Organometallic Compounds Literature Survey 1937-1958 Volume II Organic Compounds of Germanium, Tin and Lead . Springer, 2013, ISBN 978-3-662-43074-3 , pp. 43 ( limited preview in Google Book search).