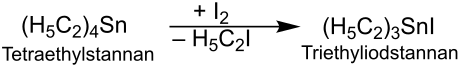Tetraethyl tin
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Tetraethyl tin | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 8 H 20 Sn | |||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 234.96 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.187 g cm −3 |
|||||||||||||||
| Melting point |
−112 ° C |
|||||||||||||||
| boiling point |
181 ° C |
|||||||||||||||
| Vapor pressure |
1.6 mbar (20 ° C) |
|||||||||||||||
| solubility |
practically insoluble in water |
|||||||||||||||
| Refractive index |
1.473 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Tetraethyltin is a chemical compound from the group of organotin compounds and has the constitutional formula Sn (C 2 H 5 ) 4 .
Extraction and presentation
Tetraethyltin can be obtained by reacting ethylmagnesium bromide with tin (IV) chloride .
properties
Tetraethyl tin is a flammable colorless liquid that is practically insoluble in water.
Reactivity
The reaction of tetraethylstannane with iodine produces triethyliodostannane :
use
Tetraethyltin is used as a reaction gas in the CVD process to produce functional layers and conductive transparent layers. It has also been used as a pesticide , fungicide, and bactericide .
It was briefly studied in the 1920s for use as an anti-knock agent for petrol , but was quickly replaced by the more effective tetraethyl lead .
safety instructions
The vapors of tetraethyl tin can form an explosive mixture with air ( flash point 53 ° C). In the body it is converted to the more toxic triethyltin . This compound became known as a neurotoxic substance in 1953/54, when 110 deaths occurred in France from the bactericidal preparation Stalinon , which contained 10% triethyltin. Triethyltin inhibits oxidative phosphorylation , glucose oxidation and the incorporation of phosphates into phospholipids and is immunotoxic.
Individual evidence
- ↑ a b c d e f g h i j k Entry on tetraethyltin in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Datasheet Tetraethyltin, 97% from Sigma-Aldrich , accessed on January 12, 2012 ( PDF ).
- ↑ GJM Van Der Kerk and JGA Luijten: Tetraethyltin In: Organic Syntheses . 36, 1956, p. 86, doi : 10.15227 / orgsyn.036.0086 ; Coll. Vol. 4, 1963, p. 881 ( PDF ).
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, p. 552, ISBN 3-342-00280-8 .
- ↑ Werner Baumann, Bettina Herberg-Liedtke: Chemicals in metal processing . Springer, 1995, ISBN 978-3-540-60094-7 , pp. 1467 ( limited preview in Google Book Search).
- ^ Vaclav Smil Distinguished Professor University of Manitoba: Transforming the Twentieth Century: Technical Innovations and Their Consequences . Oxford University Press, 2006, ISBN 0-19-803775-9 , pp. 171 ( limited preview in Google Book search).
- ↑ Jill E. Cremer (1958), "The biochemistry of organotin compounds. The conversion of tetraethyltine into triethyltine in mammals". In: Biochem J. volume 68, issue 4, pp. 685-692, PMC 1200418 (free full text).
- ↑ Wolfgang Remmele, Günter Klöppel, Hans Kreipe and Werner Paulus: Pathology: Neuropathology . Springer, 2011, ISBN 978-3-642-02323-1 , pp. 373 ( limited preview in Google Book search).
- ↑ Burkhard Madea: Praxis forensic medicine: assessment, reconstruction, assessment . 2006, ISBN 978-3-540-33719-5 , pp. 402 ( limited preview in Google Book search).