Dimethyl calcium
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
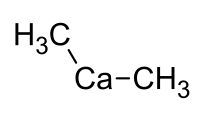
|
|||||||
| General | |||||||
| Surname | Dimethyl calcium | ||||||
| other names |
Calcium dimethyl |
||||||
| Molecular formula | C 2 H 6 Approx | ||||||
| Brief description |
white, pyrophoric solid |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 70.15 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| solubility |
practically insoluble in hydrocarbons , violent reaction with water |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Dimethyl calcium is a chemical compound from the group of organic calcium compounds with the empirical formula Ca (CH 3 ) 2 .
Extraction and presentation
Dimethyl calcium can be obtained by salt metathesis from methyl lithium and calcium bis (trimethylsilylamide) in diethyl ether .
Early reports that the synthesis should also be possible directly from methyl iodide (CH 3 I) and metallic calcium in anhydrous pyridine could not be reproduced.
properties
Dimethyl calcium is a white, amorphous and extremely pyrophoric solid that decomposes spontaneously and sometimes explosively on contact with air and water. While the compound is insoluble in hydrocarbons and diethyl ether , some solubility can be achieved in strong donor solvents such as tetrahydrofuran or tetrahydropyran . Due to the very polar nature of the Ca-C bond and the associated high reactivity with regard to ether cleavage , these solutions are only stable for a short time and at low temperatures.
Individual evidence
- ↑ a b c d e f B. M. Wolf, C. Stuhl, C. Maichle-Mössmer, R. Anwander: Dimethylcalcium In: Journal of the American Chemical Society. 140, 2018, p. 2373, doi : 10.1021 / jacs.7b12984 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ DA Payne, RT Sanderson: CALCIUM DIMETHYL, STRONTIUM DIMETHYL, AND BARIUM DIMETHYL In: Journal of the American Chemical Society. 80, 1958, p. 5324, doi : 10.1021 / ja01552a081 .
- ^ HJ Emeléus, AG Shape: Advances in Inorganic Chemistry and Radiochemistry Vol. 11 . Academic Press, 1968, ISBN 978-0-12-023611-4 , pp. 343 .
![{\ displaystyle \ mathrm {Ca [N \ {Si (CH_ {3}) _ {3} \} _ {2}] _ {2} +2 \ LiCH_ {3} \ longrightarrow Ca (CH_ {3}) _ {2} +2 \ Li [N \ {Si (CH_ {3}) _ {3} \} _ {2}]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b906eaedb287a0f1f7e57c6921bdbf955e15a9ea)