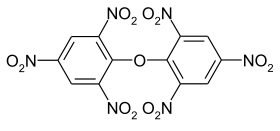
Dipicryloxide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Dipicryloxide | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 12 H 4 N 6 O 13 | |||||||||||||||
| Brief description |
pale yellow crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 440.196 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.7 g cm −3 |
|||||||||||||||
| Melting point |
269 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Dipicryloxide ( 2,4,6,2 ', 4', 6'-hexanitrodiphenyloxide ) is an explosive organic nitro compound that is derived from picric acid.
Presentation and extraction
The compound can be obtained by nitration of diphenyl ether via intermediate stages of the di-, tri-, tetra- and pentanitro substitution products. Another possibility is the formation of ethers from 2,4,6-trinitrophenol and 2,4,6-trinitrochlorobenzene in the presence of sodium hydroxide solution.
properties
Dipicryloxide is a crystalline solid. The connection is particularly explosive when dry due to impact, friction, heat and other ignition sources and is subject to the Explosives Act .
Table with important explosion-relevant properties: Oxygen balance −47.3% Nitrogen content 19.09% Lead block bulge 37.3 cm 3 g −1 Detonation velocity 7180 m · s −1 Sensitivity to impact 8 Nm
use
The compound is a very stable explosive, which compared to picric acid is less sensitive to impact, but more explosive.
Individual evidence
- ↑ a b c d e f g h i j k l Köhler, J .; Meyer, R .; Homburg, A .: Explosivstoffe , tenth, completely revised edition, Wiley-VCH, Weinheim 2008, p. 163, ISBN 978-3-527-32009-7 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Patent DE635185 (Chem. Fabr v. Heyden., 1934).
- ↑ Bandgar, BP; Dhakne, VD; Nigal, JN: Rapid synthesis of nitro substituted diaryl ethers under mild conditions in Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry 38 (1999) 111-113.
- ↑ Roth, L .; Weller, U .: Hazardous Chemical Reactions , 65th supplement, ecomed-Verlag 2011.
- ↑ Explosives Act, Appendix I, List of Explosive Substances ( BGBl. 1975 I p. 853 ), to which the law is to be applied in full.
- ↑ Sorbe: Security characteristics , edition 07/2014, ecomed Sicherheit, Hüthig Jehle Rehm GmbH publishing group.