Dynemicin A
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
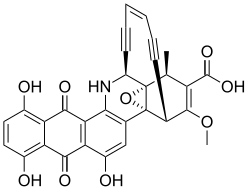
|
|||||||||||||
| General | |||||||||||||
| Surname | Dynemicin A | ||||||||||||
| Molecular formula | C 30 H 19 NO 9 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 537.48 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Dynemicin A belongs to the group of angucyclines and enediins .
properties
Dynemicin A reacts via a Bergman cyclization , whereby a para- didehydrobenzene is formed, which induces a double-strand break in B-DNA via its epoxide group . Hence, it is being studied for use as a chemotherapy drug. The strand break occurs randomly with a slight tendency to guanines . The action of Dynemicin A can be inhibited by distamycin A and anthramycin , both of which bind in the minor groove of double-stranded DNA . Therefore, Dynemicin A probably also binds in the minor groove.
synthesis
The first total synthesis for Dynemicin A was published in 1995.
Dynemicin A is formed by the actinomycetes Micromonospora chersina (from Gujarat ) and Micromonospora globosa (from Japan ) and was first isolated in the mid-1980s. The biosynthesis takes place via the polyketide route .
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ A. Beane, BR Miller, CA Parish: Internal abstraction of dynemicin A: An MD approach. In: Journal of Molecular Graphics & Modeling . Volume 74, June 2017, pp. 251-264, doi : 10.1016 / j.jmgm.2017.03.012 , PMID 28458004 .
- ↑ KC Nicolaou, JS Chen, SM Dalby: From nature to the laboratory and into the clinic. In: Bioorganic & Medicinal Chemistry . Volume 17, number 6, March 2009, pp. 2290-2303, doi : 10.1016 / j.bmc.2008.10.089 , PMID 19028103 , PMC 2665039 (free full text).
- ↑ a b c d Daniel Best: Dynemicin A, Uncialamycin and Analogues. Elsevier, 2016, ISBN 978-0-081-01086-0 . P. 1.
- ^ Andrew G. Myers, Mark E. Fraley, Norma J. Tom, Scott B. Cohen, David J. Madar: Synthesis of (+) - dynemicin A and analogs of wide structural variability: establishment of the absolute configuration of natural dynemicin A. , Chemistry & Biology (1995), 2 (1): pp. 33-43. PMID 9383401 . doi: 10.1016 / 1074-5521 (95) 90078-0 .
- ↑ Otto D. Hensens, Jose Luis Giner, Irving H. Goldberg: Biosynthesis of NCS Chrom A, the chromophore of the antitumor antibiotic neocarzinostatin , J. Am. Chem. Soc. (1989), 111 (9): pp. 3295-3299. doi: 10.1021 / ja00191a028 .
- ↑ Yoshiyuki Tokiwa, Megumi Miyoshi-Saitoh, Hisayoshi Kobayashi, Rie Sunaga, Masataka Konishi, Toshikazu Oki, Shigeo Iwasaki: Biosynthesis of dynemicin A, a 3-ene-1,5-diyne antitumor antibiotic , J. Am. Chem. Soc. (1992), 114 (11): pp. 4107-4110. doi: 10.1021 / ja00037a011 .
- ↑ Wen Liu, Joachim Ahlert, Qunjie Gao, Evelyn Wendt-Pienkowski, Ben Shen, Jon S. Thorson: Rapid PCR amplification of minimal enediyne polyketide synthase cassettes leads to a predictive familial classification model , Proceedings of the National Academy of Sciences of the United States of America (2003), 100 (21): pp. 11959-11963. PMC 218695 (free full text). PMID 14528002 . doi: 10.1073 / pnas.2034291100 .
- ↑ Qunjie Gao, Jon S. Thorson: The biosynthetic genes encoding for the production of the dynemicin enediyne core in Micromonospora chersina ATCC53710 , FEMS Microbiology Letters (2008), 282 (1): pp. 105-114. PMID 18328078 . doi: 10.1111 / j.1574-6968.2008.01112.x .
