Distamycin
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
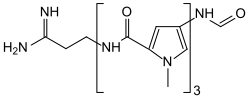
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Distamycin | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 22 H 27 N 9 O 4 • HCl | |||||||||||||||
| Brief description |
yellow solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 517.97 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
> 300 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Distamycin is a polyamide - antibiotic that to the minor groove of DNA - the double helix binds.
properties
Distamycin belongs to the class of pyrrole - amidine antibiotics and is an analog of netropsin and the lexitropsins . In contrast to netropsin, distamycin has three N-methyl-pyrrole units. Distamycin is isolated from Streptomyces distallicus . It preferentially binds to AT-rich DNA sequences and tetrads from [TGGGGT] 4 .
Distamycin inhibits transcription and increases the activity of topoisomerase II.
Various derivatives of distamycin are being investigated as alkylating agents for the treatment of tumors . Distamycin derivatives with fluorophores are used for fluorescent labeling of double-stranded DNA.
Distamycin is hygroscopic , sensitive to hydrolysis , frost and light. The extinction coefficient is 37,000 M −1 cm −1 at a wavelength of 303 nm.
Individual evidence
- ↑ a b c d e data sheet Distamycin hydrochloride from Sigma-Aldrich , accessed on November 24, 2013 ( PDF ).
- ↑ a b M. P. Barrett, CG Gemmell, CJ Suckling: Minor groove binders as anti-infective agents. In: Pharmacology & Therapeutics . Volume 139, Number 1, July 2013, pp. 12-23. doi : 10.1016 / j.pharmthera.2013.03.002 . PMID 23507040 .
- ↑ ML Kopka, C. Yoon, D. Goodsell, P. Pjura, RE Dickerson: The molecular origin of DNA-drug specificity in netropsin and distamycin. In: Proceedings of the National Academy of Sciences of the United States of America . Volume 82, Number 5, March 1985, pp. 1376-1380. PMID 2983343 . PMC 397264 (free full text).
- ↑ B. Pagano, I. Fotticchia, S. De Tito, CA Mattia, L. Mayol, E. Novellino, A. Randazzo, C. Giancola: Selective Binding of Distamycin A Derivative to G-Quadruplex Structure [d (TGGGGT)] (4). In: Journal of nucleic acids. 2010. doi : 10.4061 / 2010/247137 . PMID 20725616 . PMC 2915651 (free full text).
- ↑ P. Majumder, A. Banerjee, J. Shandilya, P. Senapati, S. Chatterjee, TK Kundu, D. Dasgupta: Minor groove binder distamycin remodels chromatin but inhibits transcription. In: PLOS ONE . Volume 8, number 2, 2013, p. E57693. doi : 10.1371 / journal.pone.0057693 . PMID 23460895 . PMC 3584068 (free full text).
- ↑ M. Fesen, Y. Pommier: Mammalian topoisomerase II activity is modulated by the DNA minor groove binder distamycin in simian virus 40 DNA. In: The Journal of Biological Chemistry . Volume 264, Number 19, July 1989, pp. 11354-11359. PMID 2544590 .
- ↑ PG Baraldi, D. Preti, F. Fruttarolo, MA Tabrizi, R. Romagnoli: Hybrid molecules between distamycin A and active moieties of antitumor agents. In: Bioorganic & Medicinal Chemistry . Volume 15, Number 1, January 2007, pp. 17-35. doi : 10.1016 / j.bmc.2006.07.004 . PMID 17081759 .
- ↑ T. Vaijayanthi, T. Bando, GN Pandian, H. Sugiyama: Progress and prospects of pyrrole-imidazole polyamide-fluorophore conjugates as sequence-selective DNA probes. In: ChemBioChem . Volume 13, Number 15, October 2012, pp. 2170-2185. doi : 10.1002 / cbic.201200451 . PMID 23023993 .
