Eletriptan
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
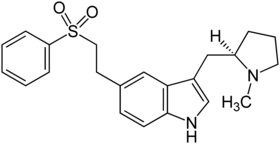
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Eletriptan | ||||||||||||||||||
| other names |
( R ) -3 - [(1-methylpyrrolidin-2-yl) methyl] -5- [2- (phenylsulfonyl) ethyl] indole |
||||||||||||||||||
| Molecular formula | C 22 H 26 N 2 O 2 S | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| Mechanism of action |
Selective 5-HT 1 receptor agonist |
||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 382.52 g · mol -1 | ||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Eletriptan (trade name Relpax ® , a product of Pfizer ) is a drug selected from the group of triptans that for the acute treatment of migraine is used. It is an agonist on the serotonin receptors of the subtype 5-HT 1B / 1D . Eletriptan was patented by Pfizer in 1992.
Clinical information
Application areas (indications)
Medicines with the active ingredient eletriptan are approved for the acute treatment of headaches in migraines with or without aura. Film-coated tablets containing 20 or 40 mg eletriptan hydrobromide are available for this application.
Contraindications (contraindications)
Eletriptan must not be used in cases of known hypersensitivity to the active substance. The active ingredient must not be used in patients with a heart attack , suspected ischemic heart disease, coronary vasospasm (Prinzmetal's angina), peripheral blood vessel diseases , moderate to severe or uncontrolled high blood pressure . Eletriptan should also not be used if you have known severe liver or kidney dysfunction or if you have a history of transient ischemic attacks. Eletriptan must not be used at the same time as ergot alkaloids and their derivatives ( e.g. ergotamine , dihydroergotamine ), as there is an increased risk of vasospasm.
Use during pregnancy and breastfeeding
Since there is insufficient experience with the use of eletriptan in pregnant women, eletriptan should only be used with caution and under strict indications during pregnancy. Animal experiments did not provide any indications of embryotoxic or teratogenic effects.
Eletriptan is principally excreted in breast milk, with about 0.02% of the total dose being found in breast milk when 80 mg of active substance is administered. Despite the small amount of eletriptan in breast milk, it should be used with caution in nursing mothers. Potential effects on the infant can be minimized by interrupting breastfeeding by expressing and discarding breast milk for at least 24 hours after taking eletriptan.
Pharmacological properties
Mechanism of action (pharmacodynamics)
Eletriptan is a selective serotonin agonist for the receptors of the subtype 5-HT 1B / 1D . In addition, Eletriptan shows a relevant affinity for 5-HT 1F receptors. These receptors are found on cerebral blood vessels and presynaptically on neurons , and their activation by eletriptan leads to a narrowing of the cerebral blood vessels that expand during a migraine attack. At the same time eletriptan leads to a reduction of the release of blutgefäßdilatierenden and nociceptive inflammatory mediators such as serotonin, calcitonin gene-related peptide (CGRP) and substance P .
Individual evidence
- ↑ a b data sheet Eletriptan hydrobromide from Sigma-Aldrich , accessed on March 29, 2011 ( PDF ).
- ↑ Entry on Eletriptan. In: Römpp Online . Georg Thieme Verlag, accessed on November 10, 2014.
- ↑ Limmroth V: mechanism of action of triptans . In: Pharmacy in our time . 31, No. 5, 2002, pp. 458-61. doi : 10.1002 / 1615-1003 (200209) 31: 5 <458 :: AID-PAUZ458> 3.0.CO; 2-G . PMID 12369163 .