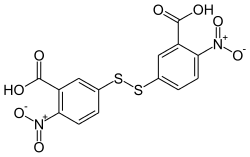
Ellman's reagent
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ellman's reagent | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 14 H 8 N 2 O 8 S 2 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 396.35 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
240–245 ° C (decomposition) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Ellman's reagent (5,5′-dithiobis-2-nitrobenzoic acid, DTNB) is a substance that is used in biochemistry to determine the amount of thiols in a sample.
properties
Thiols in biomolecules , especially in cysteine- containing proteins and peptides , react with DTNB, releasing 2-nitro-5-thiobenzoate (TNB - ), which deprotonates in neutral and basic solutions to form the yellow dye TNB 2− .
The amount of released TNB - can be quantified photometrically at a wavelength of 412 nm . The reaction takes place in a stoichiometric ratio. The extinction coefficient in dilute solutions is 14,150 M −1 cm −1 and 13,700 M −1 cm −1 in higher salt concentrations such as 6 M guanidine hydrochloride or 8 M urea. Recrystallization of the DTNB can be carried out for reproducible results .
Alternatively, 2,4-dinitrobenzenesulfonyl fluorescein or 5- (2-aminoethyl) dithio-2-nitrobenzoic acid can be used.
synthesis
Ellman produced DTNB in several steps by oxidizing 2-nitro-5-chlorobenzaldehyde to the carboxyl derivative , by generating the thiol with sodium sulfide and by oxidizing the thiol groups to disulfide bonds with iodine .
use
Ellman's reagent can be used to determine glutathione and thiol groups in proteins in purified solutions or in biological samples such as blood . The reaction with free thiol groups produces yellowish p-nitrophenyl, which can be detected spectroscopically at 412 nm.
Ellman's reagent can also be used to rapidly and specifically form inter- and intramolecular disulfide bridges between cysteines of peptides over a wide range of pH values. In solid phase synthesis, large yields are achieved with little oligomerization. The reagent binds to the solid phase on two sides. A bond is made to a thiol group. A second thiol group displaces the reagent and is thus linked to the first to form a disulfide. The thiol groups on Ellman's reagent are reduced in the process.
history
DTNB was first manufactured by George L. Ellman in 1958.
Web links
Individual evidence
- ↑ a b c data sheet 5,5′-dithiobis (2-nitrobenzoic acid) from Sigma-Aldrich , accessed on July 17, 2016 ( PDF ).
- ↑ a b c G. Ellman: A colorimetric method for determining low concentrations of mercaptans . In: Arch. Biochem. Biophys . 74, No. 2, 1958, pp. 443-450. doi : 10.1016 / 0003-9861 (58) 90014-6 .
- ^ A b Ellman GL: Tissue sulfhydryl groups . In: Arch. Biochem. Biophys. . 82, No. 1, 1959, pp. 70-77. doi : 10.1016 / 0003-9861 (59) 90090-6 . PMID 13650640 .
- ↑ Collier HB: Letter: A note on the molar absorptivity of reduced Ellman's reagent, 3-carboxylato-4-nitrothiophenolate . In: Anal. Biochem. . 56, No. 1, 1973, pp. 310-1. doi : 10.1016 / 0003-2697 (73) 90196-6 . PMID 4764694 .
- ↑ a b P. W. Riddles, RL Blakeley, B. Zerner: Reassessment of Ellman's reagent. In: Methods in enzymology. Volume 91, 1983, ISSN 0076-6879 , pp. 49-60, doi : 10.1016 / S0076-6879 (83) 91010-8 , PMID 6855597 .
- ↑ H. Maeda, H. Matsuno, M. Ushida, K. Katayama, K. Saeki, N. Itoh: 2,4-Dinitrobenzenesulfonyl fluoresceins as fluorescent alternatives to Ellman's reagent in thiol-quantification enzyme assays. In: Angewandte Chemie (International ed. In English). Volume 44, Number 19, May 2005, pp. 2922-2925, ISSN 1433-7851 . doi : 10.1002 / anie.200500114 . PMID 15818626 .
- ↑ J. Zhu, I. Dhimitruka, D. Pei: 5- (2-Aminoethyl) dithio-2-nitrobenzoate as a more base-stable alternative to Ellman's reagent. In: Organic letters. Volume 6, Number 21, October 2004, pp. 3809-3812, ISSN 1523-7060 , doi : 10.1021 / ol048404 + , PMID 15469355 .
- ↑ Sedlak J, Lindsay RH: Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent . In: Anal. Biochem. . 25, No. 1, 1968, pp. 192-205. doi : 10.1016 / 0003-2697 (68) 90092-4 . PMID 4973948 .
- ↑ Riener CK, Kada G, Gruber HJ: Quick measurement of protein sulfhydryls with Ellman's reagent and with 4,4'-dithiodipyridine . In: Anal Bioanal Chem . 373, No. 4-5, 2002, pp. 266-76. doi : 10.1007 / s00216-002-1347-2 . PMID 12110978 .
- ^ RJ Simpson: Estimation of Free Thiols and Disulfide Bonds Using Ellman's Reagent. In: CSH protocols. Volume 2008, 2008, pdb.prot4699, PMID 21356901 .
- ↑ Annis I, Chen L, Barany G: Novel Solid-Phase Reagents for Facile Formation of Intramolecular Disulfide Bridges in Peptides under Mild Conditions . In: J. Am. Soc. . 120, No. 29, 1998, pp. 7226-7238. doi : 10.1021 / ja981111p .

