Ethylhexyl compounds
| 2-ethylhexyl compounds |
|---|
 2-ethylhexanol |
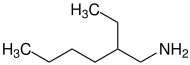 2-ethylhexylamine |
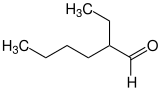 2-ethylhexanal |
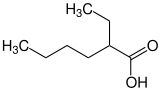 2-ethylhexanoic acid |
 2-ethylhexyl acrylate |
Under Ethylhexylverbindungen it summarizes a group of chemicals that the common structural element 2-ethylhexyl own. Occasionally the group is called the isooctyl group rather unspecifically .
Whenever the term "2-ethylhexyl compound" is used without a prefix in this article or in the scientific literature , a residue is always meant that is derived from the racemate .
Technical manufacturing
The central step of the synthesis is the aldol condensation of two molecules of butanal (as a C 4 building block) by reaction of the α-carbon atom of one molecule with the aldehyde group of the other molecule. It leads to this branched carbon structure with 8 carbon atoms.
The starting material for the production is initially propene . The propene is first reacted with carbon monoxide and hydrogen in a hydroformylation reaction to form butanal (butyraldehyde). This is followed by the aldol condensation of two molecules of butyraldehyde to form 2-ethyl-3-hydroxyhexanal .
After elimination of water and catalytic hydrogenation , 2-ethylhexanol is obtained.
Substance groups

Raw materials
Ester
- Bis (2-ethylhexyl) phthalate (DEHP)
- Bis (2-ethylhexyl) sebacate
- Bis (2-ethylhexyl) tetrabromophthalate
- Bis (2-ethylhexyl) terephthalate
- Di (2-ethylhexyl) peroxydicarbonate
- Diethylhexyl adipate
- 2-ethylhexyl acrylate
- 2-ethylhexyl salicylate
- 2-ethylhexyl 2,3,4,5-tetrabromobenzoate
- Tris (2-ethylhexyl) phosphate
- Tris (2-ethylhexyl) trimellitate
- Octyltriazone
- Octinoxate
- Octocrilen
- Bemotrizinol
- Dioctyltin bis (2-ethylhexylthioglycolate)
Other connections
Individual evidence
- ↑ a b Process for the production of 2-ethylhexanol: DE 3530839 A1 , August 29, 1985; EP 0216151 B1 , August 20, 1986.
literature
- WM Kluwe, JE Huff, HB Matthews, R. Irwin, JK Haseman: Comparative chronic toxicities and carcinogenic potentials of 2-ethylhexyl-containing compounds in rats and mice. In: Carcinogenesis . 6 (11), 1985, pp. 1577-1583. PMID 4053278 .
