Bemotrizinol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| Mixture of three stereoisomers - simplified structural formula without stereochemistry | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Bemotrizinol ( INN ) | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 38 H 49 N 3 O 5 | |||||||||||||||||||||
| Brief description |
light yellow powder or flakes |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 627.83 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.17 g cm −3 (20 ° C ) |
|||||||||||||||||||||
| Melting point |
|
|||||||||||||||||||||
| solubility |
almost insoluble in water (<10 −7 g · l −1 ), soluble in oils, such as B. Sesame oil (10%), in Miglyol® 812 (C 8 - C 12 triglyceride mixture, 14%), in Finsolv® TN (C 12 - C 15 alkyl benzoate, 25%) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Bemotrizinol ( BEMT ) is an asymmetrically substituted 1,3,5-triazine that is used as a broad spectrum UV filter in sunscreens . The triazine core substituted with functionalized aromatics is responsible for UV absorption , the hydrophobic 2-ethylhexyloxyphenol substituents for the oil solubility and water resistance in dermatological and cosmetic preparations. Bemotrizinol has a broad UV absorption spectrum with absorption maxima λmax in the UV-B range at 310 nm and in the UV-A range at 343 nm. It is mostly used in combination with other UV filters that are stabilized by BEMT.
Stereoisomerism
Bemotrizinol contains two stereocenters substituted by the same residues, hence there are three stereoisomers:
- ( R , R ) -Bemotrizinol,
- ( S , S ) -emotrizinol and
- meso -emotrizinol.
Only the mixture of these three stereoisomers is of technical and practical importance.
| Bemotrizinol stereoisomers |
|---|
 ( R , R ) stereoisomer |
 ( S , S ) stereoisomer |
 meso stereoisomer |
presentation
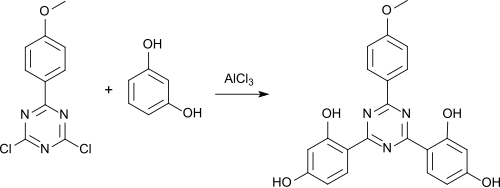
Bemotrizinol is obtained in a three-stage reaction starting from cyanuric chloride and 4-bromanisole in a total of about 57% yield. In the first stage, the Grignard compound of 4-bromanisole reacts with cyanuric chloride in tetrahydrofuran in a ratio of 1: 1 with 82% yield to give the monosubstitution product 6- (4-methoxyphenyl) -2,4-dichloro-1,3,5-triazine .
In a Friedel-Crafts acylation , the monosubstitution product with resorcinol in a xylene - sulfolane mixture in a ratio of 1: 2 is converted with resorcinol in the presence of aluminum chloride with 89% yield to the corresponding trisubstitution product 2,4-bis (2,4-dihydroxy- phenyl) -6- (4-methoxyphenyl) -1,3,5-triazine.
In the last stage, the trisubstitution product on the para hydroxyl groups of the resorcinol residues is treated with 3-bromomethylheptane ( accessible from 2-ethylhexanol and hydrobromic acid ) in a ratio of 1: 2.2 in 2-methoxyethanol (methylcellosolve) and sodium hydroxide solution in 78% Yield alkylated.
After purification by chromatography on silica gel , a tough, slightly yellow resin is obtained which crystallizes out after a few weeks. The addition of the seed crystals accelerates the crystallization of the end product. The preparation of the pure crystalline bemotrizinol (> 96.5% content) raises considerable problems, since stubbornly adhering solvent residues and by-products, as they are also listed in the USP monograph, hinder the crystallization.
properties
Bemotrizinol is a light yellow, odorless solid that is insoluble in water and other polar solvents. Bemotrizinol dissolves well in oils such as those used for cosmetic preparations, in particular sunscreens.
As a hydroxyphenyltriazine, bemotrizinol is extremely photostable and, unlike other sunscreens, does not form any toxic or allergenic photodegradation products.
No negative data are available on the reproductive and developmental toxicity of bemotrizinol.
use
The broad-spectrum UV absorber bemotrizinol is a strong UV-B absorber with a specific extinction coefficient of 819 and a UV-A absorption maximum of 340 nm (under standard conditions in ethanol ), with the UV-A / UV-B ratio of 0 , 73 indicates a likewise high UV-A absorption.
BEMT is in because of its high absorption activity, its synergistic effect with a significant increase of the Sun Protection Factor (engl. Sun protection factor SPF booster) and its stabilizing properties to other UV absorbers in concentrations of up to 10% (in Japan up to 3% ) used in combination with other UV filters in sunscreens. The water-insoluble BEMT can be embedded in a water-dispersible polymethyl methacrylate matrix in concentrations of up to 20% and is thus available as a pale yellow liquid dispersion (Tinosorb® Aqua), which can be incorporated into oil-in-water emulsions.
In the USA, sunscreens, as OTC drugs, are subject to the regulations of the US FDA, which has not approved any new active ingredients for sunscreens for more than ten years. Despite the so-called Time and Extent Applications (TEA) introduced in 2002 to accelerate the approval process and the so-called Sunscreen Innovation Act (SIA) enacted at the end of 2014, like seven other modern sunscreens approved in Europe and other regions, Bemotrizinol is in the for the time being USA not available.
Bemotrizinol is manufactured and sold by BASF SE (under the brand name Tinosorb® S) and Ashland Inc. (Escalol® S).
Individual evidence
- ↑ Entry on BIS-ETHYLHEXYLOXYPHENOL METHOXYPHENYL TRIAZINE in the CosIng database of the EU Commission, accessed on December 29, 2019.
- ↑ a b c data sheet Bemotrizinol from Sigma-Aldrich , accessed on March 18, 2016 ( PDF ).
- ↑ a b c d e LGC, safety data sheet, bemotrizinol , archive link ( Memento of the original from March 25, 2016 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ a b c Patent EP0775698A1 : Bis-resorcinyl-triazines as UV absorbers. Applied on November 14, 1996 , published on May 28, 1997 , Applicant: Ciba Specialty Chemicals Holding Inc., Inventors: D. Hüglin, E. Borsos, H. Luther, B. Herzog, F. Bachmann.
- ↑ a b c d Attachment 2, Tinosorb® S Bis-Ethylhexyloxyphenol Methoxyphenol Triazine (BEMT), http://www.fda.gov/ohrms/dockets/dailys/00/Sep00/090600/cp00001_attachment_02.pdf
- ^ SQ Wang: The Making of an Effective Sunscreen. (No longer available online.) In: mskcc.org. December 9, 2014, formerly in the original ; accessed on March 18, 2016 . ( Page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice.
- ^ B. Herzog, M. Wehrle, K. Quass: Photostability of UV absorber systems in sunscreens . In: Photochem. Photophys. tape 85 , 2009, p. 869-878 , doi : 10.1111 / j.1751-1097.2009.00544.x .
- ↑ Authorized USP Pending Monograph, Version 2, Bemotrizinol, February 1, 2009, archive link ( Memento of the original from January 29, 2013 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ CG Benevenuto, LO Guerra, LR Gaspar: Combination of retinyl palmitate and UV-filters: phototoxic risk assessment based on photostability and in vitro and in vivo phototoxicity assays . In: Eur. J. Pharm. Sci. tape 68 , 2015, p. 127-136 , doi : 10.1016 / j.ejps.2014.12.007 .
- ↑ Center of Endocrine Disrupters, Assessment of the endocrine disruptive potential of 23 UV-filters (j.no. MST-656-00150), M. Axelstad, U. Hass, K. Lund Kinnberg, P. Bjerregaard ( PDF ( Memento des Originals from March 4, 2016 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. ).
- ↑ J. Ashby, H. Tinwell, K. Twomey, PA Lefevre: Lack of Binding to Isolated estrogen or androgen Receptors, and Inactivity in the Immature Rat uterotrophic assay of the Ultraviolet Sunscreen filter Tinosorb M-Active and Tinosorb S . In: Regul. Toxicol. Pharmacol. tape 34 , no. 3 , 2001, p. 287-291 , doi : 10.1006 / rtph . 2001.1511 .
- ↑ NA Shaath: SPF Boosters & Photo Stability of ultraviolet filter. HAPPI, October 2007.
- ↑ BASF, Cross-Reference List of all UV Filters used in the BASF Sunscreen Simulator ( PDF ( Memento of the original from May 29, 2015 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. ).
- ↑ BASF Personal Care, Technical Information, UV Filters, November 2011, http://dewolfchem.com/wp-content/uploads/2013/08/TDS-zcote.pdf
- ↑ a b M. S. Reisch: After More Than A Decade, FDA Still Won't Allow New Sunscreens . In: Chemical & Engineering News . tape 93 , no. 20 , 2015, p. 10-15 ( acs.org ).
- ↑ Time and Extent Application, Bemotrizinol, submitted on April 11, 2005, http://www.fda.gov/ohrms/dockets/dockets/05n0446/05n-0446-bkg0001-08-Tab-02-vol3.pdf
- ↑ US Food and Drug Administration, Sunscreen Innovation Act (SIA), http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/ucm434782.htm